326:
20:
286:
132:
in liquid soaps and detergents used to clean wool, as surface cleaners, and as active ingredients in laundry detergents, shampoos and conditioners. They can also be found in household products such as toothpaste, antacids, cosmetics and foods. Generally they are found in consumer products at
203:
chain of the alcohol will be linear. If derived using the oxo process, a low level of branching will appear usually with a methyl or ethyl group at the C-2 position, containing even and odd amounts of alkyl chains. These alcohols react with
488:
from 10-11 to 10-15 hPa). Soil sorption is proportional to carbon chain length, with a length of 14 and more having the highest sorption rate. Soil concentrations have been found to vary from 0.0035 to 0.21 milligrams per kilogram
442:
The primary disposal of alkyl sulfate from used commercial products is wastewater. The concentration of alkylsulfates in effluent from waste water treatment plants (WWTP) has been measured at 10 micrograms per litre
829:
Wibbertmann, A; Mangelsdorf, I.; Gamon, K.; Sedlak, R. (2011). "Toxicological properties and risk assessment of the anionic surfactants category: Alkyl sulfates, primary alkane sulfonates, and α-Olefin sulfonate".
531:
Eduard
Smulders, Wolfgang von Rybinski, Eric Sung, Wilfried Rähse, Josef Steber, Frederike Wiebel, Anette Nordskog "Laundry Detergents" in Ullmann's Encyclopedia of Industrial Chemistry 2007, Wiley-VCH, Weinheim.
447:
10 oz/cu in) and lower. Alkyl sulfates biodegrade easily, even starting likely before reaching the WWTP. Once at the treatment plant, they are rapidly removed by
411:
sulfate and an additional metabolite. The highest irritant of the alkyl sulfates is sodium laurylsulfate, with the threshold before irritation at a concentration of 20%.
514:
372:
775:
549:
Klaus Noweck, Wolfgang
Grafahrend, "Fatty Alcohols" in Ullmann’s Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim.
371:
Sulfate is an inert anion, so nature activates it by the formation of ester derivative of adenosine 5'-phosphosulfate (APS) and
639:"Sulfate ester formation and hydrolysis: a potentially important yet often ignored aspect of the sulfur cycle of aerobic soils"
484:
In terms of thermal stability, alkyl sulfates degrade well before reaching their boiling point due to low vapor pressure (for C
583:
758:
811:
451:. Invertebrates were found to be the most-sensitive trophic group to alkyl sulfates. Sodium laurylsulfate tested on
391:
Because they are widely used in commercial products, the safety aspects of organosulfates are heavily investigated.
349:
455:, a protozoan, was found to have the lowest effect value with the 20 h-EC5 being 0.75 milligrams per litre (2.7
379:
of sulfur compounds required for life. The formation and hydrolysis of natural sulfate esters are catalyzed by
133:
concentrations ranging from 3-20%. In 2003 approximately 118,000 t/a of alkyl sulfates were used in the US
602:
Cleland, W. Wallace; Hengge, Alvan C. (2006). "Enzymatic
Mechanisms of Phosphate and Sulfate Transfer".
274:
188:
120:(also known as sulfuric acid mono dodecyl ester sodium salt) and related potassium and ammonium salts.
776:"SIDS Initial Assessment Profile. SIAM 25: Alkyl Sulfates, Alkane Sulfonates, and α-Olefin sulfonates"
812:"SIDS Initial Assessment Profile SIAM 25: Alkyl Sulfates, Alkane Sulfonates, and α-Olefin sulfonates"
270:
336:
Several classes of sulfate esters exist in nature. Especially common are sugar derivatives such as
870:
753:
M. T. Madigan, J. M. Martinko, J. Parker "Brock
Biology of Microorganisms" Prentice Hall, 1997.
173:
31:
142:
117:
415:
in consumer products are typically mixed, reducing likelihood of irritation. According to
8:
786:
341:
205:
78:
469:
740:
663:
638:
847:
754:
709:
668:
619:
579:
357:
310:
297:-OR'. They are prepared from sulfuric acid and the alcohol. The main examples are
839:
736:
699:
658:
650:
611:
571:
550:
533:
314:
302:
43:
39:
575:
554:
537:
375:(PAPS). Many organisms utilize these reactions for metabolic purposes or for the
843:
654:
337:
298:
235:
325:
448:
169:
96:) although many are not prepared in this way. Many sulfate esters are used in
864:
828:
419:
406, alkyl sulfates in animal studies were not found to be skin sensitizers.
192:
184:
165:
82:
70:
24:
851:
713:
704:
687:
623:
431:
376:
196:
162:
234:
Alternatively, alcohols can be converted to the half sulfate esters using
183:
Alkylsulfate can be produced from alcohols, which in turn are obtained by
672:
399:
Alkyl sulfates if ingested are well-absorbed and are metabolized into a C
200:
412:
129:
105:
615:
427:
423:
380:
177:
97:
727:
Scherer, H.W. (2001). "Sulphur in crop production — invited paper".
353:
416:
363:
A major portion of soil sulfur is in the form of sulfate esters.
345:
329:
306:
109:
101:
66:
352:
of some proteins entail sulfation, often at the phenol group of
19:
285:
161:
Na. Also common in consumer products are the sulfate esters of
293:
A less common family of organosulfates have the formula RO-SO
113:
74:
688:"The Biology and Enzymology of Protein Tyrosine O-Sulfation"
773:
515:"Surfactants, household detergents and their raw materials"
478:
422:
Laboratory studies have not found alkyl sulfates to be
332:
is a medication and naturally occurring organosulfate.
459:
10 lb/cu in). Chronic exposure tests with C
434:. No long-term reproductive effects have been found.
23:
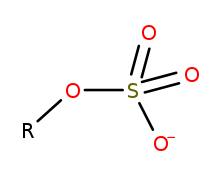
Generic structure of an organosulfate, where R is an
116:
to neutralize the sulfate group. Examples include:
112:group (containing an anion) and either a cation or
269:Specialized organosulfates can be prepared by the
187:of animal or vegetable oils and fats or using the
16:Organic compounds of the form R–O–SO₃ (charge –1)
862:
636:
568:Ullmann's Encyclopedia of Industrial Chemistry
360:, a latent precursor to the hormone estrogen.
601:
313:. These compounds are potentially dangerous
128:Alkyl sulfates are commonly used as anionic
317:. Dialkylsulfates do not occur in nature.
136:
559:
703:
662:
597:
595:
566:Holmberg, Krister (2019). "Surfactants".
320:
769:
767:
565:
324:
284:
18:
726:
863:
832:Ecotoxicology and Environmental Safety
592:
305:, colourless liquids that are used as
199:feedstock or the Ziegler process, the
764:
685:
373:3'-phosphoadenosine-5'-phosphosulfate
774:SDA/Alkylsulfate Consortium (2007).
473:found the highest toxicity is with C
264:
512:
13:
280:
73:. All organosulfates are formally
14:
882:
356:residues. A steroidal sulfate is
350:Post-translational modifications
822:
803:
692:Journal of Biological Chemistry
394:
289:Structure of a diorganosulfate.
123:
104:. Alkyl sulfates consist of a
747:
720:
679:
630:
543:
525:
506:
437:
1:
741:10.1016/S1161-0301(00)00082-4
576:10.1002/14356007.a25_747.pub2
555:10.1002/14356007.a10_277.pub2
538:10.1002/14356007.a08_315.pub2
519:CEH Marketing Research Report
500:
366:
69:group and the R group is any
844:10.1016/j.ecoenv.2011.02.007
729:European Journal of Agronomy
655:10.1128/br.40.3.698-721.1976
7:
168:such as those derived from
108:hydrocarbon chain, a polar
10:
887:
785:. Helsinki. Archived from
637:J. W. Fitzgerald (1976).
386:
271:Elbs persulfate oxidation
809:
686:Moore, Kevin L. (2003).
344:, and the anticoagulant
176:, an ingredient in some
137:Synthetic organosulfates
643:Bacteriological Reviews
383:(aka sulfohydrolases).
705:10.1074/jbc.R300008200
481:was 0.045 mg/L).
467:with the invertebrate
333:
321:Natural sulfate esters
290:
275:Boyland–Sims oxidation
174:sodium laureth sulfate
100:, and some are useful
32:organosulfur chemistry
27:
328:
288:
145:, with the formula CH
143:sodium lauryl sulfate
118:sodium lauryl sulfate
22:
513:CEH (October 2004).
195:. If produced from
141:A common example is
698:(27): 24243–24246.
497:10 oz/lb) dw.
342:chondroitin sulfate
273:of phenols and the
206:chlorosulfuric acid
46:with the structure
470:Ceriodaphnia dubia
334:
291:
28:
616:10.1021/cr050287o
585:978-3-527-30673-2
570:. pp. 1–56.
358:estradiol sulfate
315:alkylating agents
311:organic synthesis
265:Laboratory routes
172:. An example is
42:sharing a common
40:organic compounds
878:
856:
855:
838:(5): 1089–1106.
826:
820:
819:
810:DE/ICCA (2009).
807:
801:
800:
798:
797:
791:
780:
771:
762:
751:
745:
744:
724:
718:
717:
707:
683:
677:
676:
666:
634:
628:
627:
610:(8): 3252–3278.
604:Chemical Reviews
599:
590:
589:
563:
557:
547:
541:
529:
523:
522:
510:
496:
492:
458:
453:Uronema parduczi
446:
303:dimethyl sulfate
260:
230:
95:
64:
57:
56:
55:
52:
44:functional group
886:
885:
881:
880:
879:
877:
876:
875:
861:
860:
859:
827:
823:
808:
804:
795:
793:
789:
778:
772:
765:
752:
748:
725:
721:
684:
680:
635:
631:
600:
593:
586:
564:
560:
548:
544:
530:
526:
511:
507:
503:
494:
490:
487:
476:
466:
462:
456:
444:
440:
410:
406:
402:
397:
389:
369:
338:keratan sulfate
323:
299:diethyl sulfate
296:
283:
281:Dialkylsulfates
267:
258:
254:
250:
246:
242:
236:sulfur trioxide
228:
224:
220:
216:
212:
189:Ziegler process
160:
156:
152:
148:
139:
126:
94:
90:
86:
71:organic residue
63:
59:
53:
50:
49:
47:
38:are a class of
17:
12:
11:
5:
884:
874:
873:
871:Organosulfates
858:
857:
821:
802:
763:
746:
719:
678:
649:(3): 698–721.
629:
591:
584:
558:
542:
524:
504:
502:
499:
485:
474:
464:
460:
449:biodegradation
439:
436:
408:
404:
400:
396:
393:
388:
385:
368:
365:
322:
319:
294:
282:
279:
266:
263:
262:
261:
256:
252:
248:
244:
232:
231:
226:
222:
218:
214:
170:lauryl alcohol
166:fatty alcohols
158:
154:
150:
146:
138:
135:
125:
122:
92:
88:
61:
36:organosulfates
15:
9:
6:
4:
3:
2:
883:
872:
869:
868:
866:
853:
849:
845:
841:
837:
833:
825:
817:
813:
806:
792:on 2016-03-03
788:
784:
777:
770:
768:
760:
759:0-13-520875-0
756:
750:
742:
738:
735:(2): 81–111.
734:
730:
723:
715:
711:
706:
701:
697:
693:
689:
682:
674:
670:
665:
660:
656:
652:
648:
644:
640:
633:
625:
621:
617:
613:
609:
605:
598:
596:
587:
581:
577:
573:
569:
562:
556:
552:
546:
539:
535:
528:
520:
516:
509:
505:
498:
482:
480:
472:
471:
454:
450:
435:
433:
429:
425:
420:
418:
414:
392:
384:
382:
378:
374:
364:
361:
359:
355:
351:
347:
343:
339:
331:
327:
318:
316:
312:
308:
304:
300:
287:
278:
277:of anilines.
276:
272:
241:
240:
239:
237:
211:
210:
209:
207:
202:
198:
194:
193:oxo synthesis
190:
186:
185:hydrogenation
181:
179:
175:
171:
167:
164:
144:
134:
131:
121:
119:
115:
111:
107:
103:
99:
84:
83:sulfuric acid
80:
77:derived from
76:
72:
68:
45:
41:
37:
33:
26:
25:organyl group
21:
835:
831:
824:
815:
805:
794:. Retrieved
787:the original
782:
749:
732:
728:
722:
695:
691:
681:
646:
642:
632:
607:
603:
567:
561:
545:
527:
518:
508:
483:
468:
452:
441:
432:carcinogenic
421:
398:
395:Human Health
390:
377:biosynthesis
370:
362:
335:
292:
268:
233:
197:oleochemical
182:
140:
127:
124:Applications
35:
29:
438:Environment
413:Surfactants
201:hydrocarbon
191:or through
163:ethoxylated
130:surfactants
106:hydrophobic
796:2011-10-14
501:References
381:sulfatases
367:Metabolism
251:OH → RCH
221:OH → RCH
98:detergents
65:core is a
783:OECD SIDS
493:10 to 3.4
428:mutagenic
424:genotoxic
178:cosmetics
865:Category
852:21463896
714:12730193
624:16895327
354:tyrosine
307:reagents
102:reagents
79:alcohols
417:OECD TG
346:heparin
330:Heparin
229:H + HCl
217:H + RCH
110:sulfate
67:sulfate
58:. The
850:
757:
712:
673:791238
671:
664:413977
661:
622:
582:
387:Safety
75:esters
48:R−O−SO
790:(PDF)
779:(PDF)
247:+ RCH
114:amine
848:PMID
816:OECD
755:ISBN
710:PMID
669:PMID
620:PMID
580:ISBN
489:(5.6
486:8-18
479:NOEC
463:to C
443:(5.8
407:or C
301:and
213:ClSO
81:and
840:doi
737:doi
700:doi
696:278
659:PMC
651:doi
612:doi
608:106
572:doi
551:doi
534:doi
430:or
403:, C
348:.
309:in
255:OSO
225:OSO
157:OSO
149:(CH
30:In
867::
846:.
836:74
834:.
814:.
781:.
766:^
733:14
731:.
708:.
694:.
690:.
667:.
657:.
647:40
645:.
641:.
618:.
606:.
594:^
578:.
517:.
475:14
465:18
461:12
426:,
340:,
243:SO
238::
208::
180:.
155:11
91:SO
60:SO
34:,
854:.
842::
818:.
799:.
761:.
743:.
739::
716:.
702::
675:.
653::
626:.
614::
588:.
574::
553::
540:.
536::
521:.
495:×
491:×
477:(
457:×
445:×
409:5
405:4
401:3
295:2
259:H
257:3
253:2
249:2
245:3
227:3
223:2
219:2
215:3
159:3
153:)
151:2
147:3
93:4
89:2
87:H
85:(
62:4
54:3
51:−
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.