650:
4426:
4450:
4571:
658:
1105:
786:
40:
720:
4462:
4438:
345:
253:
to the other through an aqueous medium. Faraday did not know the nature of these species, but he knew that since metals dissolved into and entered a solution at one electrode and new metal came forth from a solution at the other electrode; that some kind of substance has moved through the solution in
769:
An alternative (and acceptable) way of showing a molecule/atom with multiple charges is by drawing out the signs multiple times, this is often seen with transition metals. Chemists sometimes circle the sign; this is merely ornamental and does not alter the chemical meaning. All three representations
371:
Anion (−) and cation (+) indicate the net electric charge on an ion. An ion that has more electrons than protons, giving it a net negative charge, is named an anion, and a minus indication "Anion (−)" indicates the negative charge. With a cation it is just the opposite: it has fewer electrons than
1344:. When a highly electropositive metal is combined with a highly electronegative nonmetal, the extra electrons from the metal atoms are transferred to the electron-deficient nonmetal atoms. This reaction produces metal cations and nonmetal anions, which are attracted to each other to form a
915:
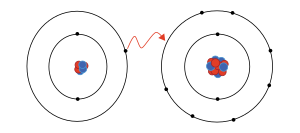
atom, Na, has a single electron in its valence shell, surrounding 2 stable, filled inner shells of 2 and 8 electrons. Since these filled shells are very stable, a sodium atom tends to lose its extra electron and attain this stable configuration, becoming a sodium cation in the process
321:, determine the size of atoms and molecules that possess any electrons at all. Thus, anions (negatively charged ions) are larger than the parent molecule or atom, as the excess electron(s) repel each other and add to the physical size of the ion, because its size is determined by its
284:
into paired charged particles when dissolved, for which he would win the 1903 Nobel Prize in
Chemistry. Arrhenius' explanation was that in forming a solution, the salt dissociates into Faraday's ions, he proposed that ions formed even in the absence of an electric current.
293:
Ions in their gas-like state are highly reactive and will rapidly interact with ions of opposite charge to give neutral molecules or ionic salts. Ions are also produced in the liquid or solid state when salts interact with solvents (for example, water) to produce
174:. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and a positive ion. Ions are also created by chemical interactions, such as the
1300:
that arises from the mutual attraction of oppositely charged ions. Ions of like charge repel each other, and ions of opposite charge attract each other. Therefore, ions do not usually exist on their own, but will bind with ions of opposite charge to form a
1036:
This driving force is what causes sodium and chlorine to undergo a chemical reaction, wherein the "extra" electron is transferred from sodium to chlorine, forming sodium cations and chloride anions. Being oppositely charged, these cations and anions form
306:
changes as the ions move away from each other to interact with the liquid. These stabilized species are more commonly found in the environment at low temperatures. A common example is the ions present in seawater, which are derived from dissolved salts.
661:
Avalanche effect between two electrodes. The original ionization event liberates one electron, and each subsequent collision liberates a further electron, so two electrons emerge from each collision: the ionizing electron and the liberated
553:. In both inorganic and organic chemistry (including biochemistry), the interaction of water and ions is often relevant for understanding properties of systems; an example of their importance is in the breakdown of adenosine triphosphate (
325:. Cations are smaller than the corresponding parent atom or molecule due to the smaller size of the electron cloud. One particular cation (that of hydrogen) contains no electrons, and thus consists of a single proton –
367:. Hydrogen forms the only charge-+1 cation that has no electrons, but even cations that (unlike hydrogen) retain one or more electrons are still smaller than the neutral atoms or molecules from which they are derived.
793:
ion. The oxidation state of the metal is shown as superscripted Roman numerals, whereas the charge of the entire complex is shown by the angle symbol together with the magnitude and sign of the net charge.
705:
to multiply the effect of the original ionizing event by means of a cascade effect whereby the free electrons are given sufficient energy by the electric field to release further electrons by ion impact.
375:
Since the electric charge on a proton is equal in magnitude to the charge on an electron, the net electric charge on an ion is equal to the number of protons in the ion minus the number of electrons.
3037:
310:
As charged objects, ions are attracted to opposite electric charges (positive to negative, and vice versa) and repelled by like charges. When they move, their trajectories can be deflected by a
682:. The original ionization event in these instruments results in the formation of an "ion pair"; a positive ion and a free electron, by ion impact by the radiation on the gas molecules. The
1094:
1031:
965:
1338:
just a few electrons short of a stable configuration. As such, they have the tendency to gain more electrons in order to achieve a stable configuration. This tendency is known as
824:
of an element, whereas the superscripted Indo-Arabic numerals denote the net charge. The two notations are, therefore, exchangeable for monatomic ions, but the Roman numerals
828:
be applied to polyatomic ions. However, it is possible to mix the notations for the individual metal centre with a polyatomic complex, as shown by the uranyl ion example.
428:), meaning "up") is an ion with more electrons than protons, giving it a net negative charge (since electrons are negatively charged and protons are positively charged).
111:, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons.
506:
and they differ in relative size: "Cations are small, most of them less than 10 m (10 cm) in radius. But most anions are large, as is the most common Earth anion,
739:
for an ion, its net charge is written in superscript immediately after the chemical structure for the molecule/atom. The net charge is written with the magnitude
3385:
3021:
2941:
2882:
2823:
234:, meaning "up"). They are so called because ions move toward the electrode of opposite charge. This term was introduced (after a suggestion by the English
1279:, which is why, in general, metals will lose electrons to form positively charged ions and nonmetals will gain electrons to form negatively charged ions.
1209:, rather than gaining or losing electrons. This allows the molecule to preserve its stable electronic configuration while acquiring an electrical charge.
3111:
889:, and so do not participate in this kind of chemical interaction. The process of gaining or losing electrons from a neutral atom or molecule is called
1267:. On the other side of the periodic table, chlorine has seven valence electrons, so in ionized form it is commonly found with one gained electron, as
3320:
3261:
3446:
885:(the outer-most electron shell) in an atom. The inner shells of an atom are filled with electrons that are tightly bound to the positively charged
3521:
974:
atom, Cl, has 7 electrons in its valence shell, which is one short of the stable, filled shell with 8 electrons. Thus, a chlorine atom tends to
846:
ion. Just like uncharged radicals, radical ions are very reactive. Polyatomic ions containing oxygen, such as carbonate and sulfate, are called
363:, with its loosely held two-electron cloud, has a larger radius than the neutral atom, which in turn is much larger than the bare proton of the
1324:, which rarely form chemical compounds). Metals are characterized by having a small number of electrons in excess of a stable, closed-shell
1257:
is exhausted of electrons. For this reason, ions tend to form in ways that leave them with full orbital blocks. For example, sodium has one
3622:
3479:
1271:. Caesium has the lowest measured ionization energy of all the elements and helium has the greatest. In general, the ionization energy of
3627:
1227:
required to detach an electron in its lowest energy state from an atom or molecule of a gas with less net electric charge is called the
2937:
2878:
2819:
549:. Atoms in their ionic state may have a different color from neutral atoms, and thus light absorption by metal ions gives the color of
3353:
2798:
1328:. As such, they have the tendency to lose these extra electrons in order to attain a stable configuration. This property is known as
1205:
Due to the instability of radical ions, polyatomic and molecular ions are usually formed by gaining or losing elemental ions such as
904:
is the transfer of electrons between atoms or molecules. This transfer is usually driven by the attaining of stable ("closed shell")
4500:
3605:
1253:
Each successive ionization energy is markedly greater than the last. Particularly great increases occur after any given block of
3649:
2857:
2916:
3237:
3188:
3151:
3001:
653:
Schematic of an ion chamber, showing drift of ions. Electrons drift faster than positive ions due to their much smaller mass.
611:, the disruption of this gradient contributes to cell death. This is a common mechanism exploited by natural and artificial
3661:
3600:
3082:
1313:. In ionic compounds there arise characteristic distances between ion neighbours from which the spatial extension and the
254:
a current. This conveys matter from one place to the other. In correspondence with
Faraday, Whewell also coined the words
3514:
3290:
2761:
4771:
4658:
3420:
3031:
472:
416:
3381:
4811:
487:
There are additional names used for ions with multiple charges. For example, an ion with a −2 charge is known as a
3172:
4821:
4466:
1051:
802:
359:. Removal of the electron gives a cation (left), whereas the addition of an electron gives an anion (right). The
4749:
4345:
3507:
3282:
3103:
988:
922:
145:
If only a + or - is present, it indicates a +1 or -1 charge (2+ indicates charge +2, 2- indicates charge -2).
4831:
3788:
3542:
578:
1136:
Polyatomic and molecular ions are often formed by the gaining or losing of elemental ions such as a proton,
817:
or Fe III (Fe I for a neutral Fe atom, Fe II for a singly ionized Fe ion). The Roman numeral designates the
4493:
545:
and are responsible for diverse phenomena from the luminescence of the Sun to the existence of the Earth's
4986:
4796:
4663:
4065:
3552:
3342:
3316:
3257:
2736:
3443:
4836:
4806:
4791:
4759:
4442:
3991:
3962:
3942:
3895:
3229:
3058:
751:. However, the magnitude of the charge is omitted for singly charged molecules/atoms; for example, the
499:
is a neutral molecule with positive and negative charges at different locations within that molecule.
4851:
4598:
3580:
3412:
666:
The ionizing effect of radiation on a gas is extensively used for the detection of radiation such as
3210:
694:
107:
is considered to be negative by convention and this charge is equal and opposite to the charge of a
4991:
4866:
4764:
4335:
4251:
3890:
1325:
905:
281:
17:
4871:
4816:
4801:
4754:
4734:
4693:
4668:
4648:
4628:
4486:
4273:
4184:
4147:
4031:
3957:
3778:
3761:
3704:
3312:
3253:
175:
4960:
4861:
4191:
4179:
4070:
3935:
3709:
3575:
3468:
2315:
1335:
1176:
979:
634:
554:
3143:
3133:
280:
put forth, in his 1884 dissertation, the explanation of the fact that solid crystalline salts
4826:
4722:
4643:
4340:
4237:
4222:
4152:
4075:
3907:
3857:
3766:
3691:
3590:
2787:
582:
484:), meaning "down") is an ion with fewer electrons than protons, giving it a positive charge.
607:
As signalling and metabolism in organisms are controlled by a precise ionic gradient across
4704:
4688:
4673:
4509:
4330:
4285:
4060:
3880:
3810:
3567:
3547:
3176:
2782:
2192:
1320:
The most common type of ionic bonding is seen in compounds of metals and nonmetals (except
1263:
in its outermost shell, so in ionized form it is commonly found with one lost electron, as
1218:
698:
31:
3346:
8:
4856:
4841:
4776:
4744:
4739:
4555:
4353:
4307:
4232:
4205:
4103:
4085:
4038:
3976:
3872:
3852:
3721:
3716:
3617:
3340:
3139:
1199:
842:
135:
3180:
4846:
4717:
4528:
4430:
4396:
4258:
4227:
4108:
4050:
3748:
3731:
3726:
3681:
3644:
3634:
3595:
3198:
2756:
2605:
2218:
1511:
1109:
702:
683:
4570:
908:. Atoms will gain or lose electrons depending on which action takes the least energy.
525:(for ions that respectively travel to the anode and cathode during electrolysis) were
4920:
4608:
4449:
4411:
4376:
4359:
4297:
4215:
4210:
4138:
4123:
4093:
4014:
3981:
3952:
3947:
3922:
3912:
3832:
3820:
3699:
3612:
3426:
3416:
3377:
3233:
3184:
3147:
3027:
2997:
2993:
2959:
2931:
2872:
2813:
1340:
1330:
837:
574:
4638:
2838:
4786:
4781:
4593:
4533:
4454:
4371:
4026:
3885:
3862:
3815:
3756:
2897:
2264:
2241:
1346:
1259:
1195:
882:
736:
586:
440:
387:
277:
179:
62:
856:. If the charge in an organic ion is formally centred on a carbon, it is termed a
4653:
4580:
4312:
4268:
4263:
4157:
4133:
3967:
3930:
3783:
3773:
3656:
3450:
2902:
2843:
1302:
1175:. Ammonia and ammonium have the same number of electrons in essentially the same
1042:
820:
530:
514:
is occupied by the anion and that the cations fit into the spaces between them."
317:
Electrons, due to their smaller mass and thus larger space-filling properties as
242:
238:
100:
4889:
4543:
4196:
4174:
4169:
4164:
4119:
4115:
4098:
4055:
3986:
3847:
3842:
3827:
3639:
3557:
3078:
2091:
1759:
1254:
886:
798:
667:
601:
360:
322:
311:
223:
211:
183:
171:
157:
139:
3286:
852:. Molecular ions that contain at least one carbon to hydrogen bond are called
134:(hydroxide ion)). Opposite electric charges are pulled towards one another by
4981:
4975:
4523:
4401:
4290:
4246:
3971:
3805:
3800:
3793:
3671:
2792:
1850:
1380:
1297:
1182:
Ammonia can also lose an electron to gain a positive charge, forming the ion
671:
638:
608:
372:
protons, giving it a net positive charge, hence the indication "Cation (+)".
348:
167:
3430:
686:
is the simplest of these detectors, and collects all the charges created by
4727:
4683:
4618:
4560:
4538:
4278:
4128:
4043:
4019:
4009:
4001:
3902:
3837:
3736:
3585:
2751:
2583:
2403:
2301:
2144:
1314:
1129:
623:
616:
590:
503:
318:
4955:
4940:
4678:
4603:
3676:
2731:
2721:
2535:
2381:
1156:
1113:
858:
597:
4478:
3499:
1179:, but ammonium has an extra proton that gives it a net positive charge.
649:
4899:
4633:
4613:
4302:
2513:
2166:
2014:
1288:
1038:
619:
566:
557:), which provides the energy for many reactions in biological systems.
546:
496:
191:
4945:
4935:
4925:
4894:
4550:
4364:
3666:
3531:
2741:
2447:
2287:
2100:
1957:
1790:
1701:
1656:
1591:
1417:
1389:
1321:
1276:
1239:
th ionization energy of an atom is the energy required to detach its
901:
897:
864:
675:
657:
627:
250:
131:
119:
1104:
126:
is a negatively charged ion with more electrons than protons. (e.g.
118:
is a positively charged ion with fewer electrons than protons (e.g.
4623:
4386:
2746:
2561:
2425:
2122:
2067:
2045:
1999:
1901:
1887:
1768:
1160:
971:
848:
612:
550:
492:
356:
235:
160:
153:
127:
104:
96:
48:
881:
Monatomic ions are formed by the gain or loss of electrons to the
337:"Anion" redirects here. Not to be confused with the quasiparticle
4930:
4406:
2695:
2668:
2653:
2634:
2491:
2469:
2359:
2337:
1985:
1943:
1915:
1873:
1859:
1577:
1431:
1141:
809:(positively doubly charged) example seen above is referred to as
785:
728:
573:
or temperature. These are used in a multitude of devices such as
570:
511:
488:
303:
262:
44:
1334:. Non-metals, on the other hand, are characterized by having an
719:
298:, which are more stable, for reasons involving a combination of
39:
2726:
1929:
1686:
1671:
1403:
1272:
1224:
912:
790:
752:
679:
542:
507:
364:
352:
299:
108:
3281:
1194:. However, this ion is unstable, because it has an incomplete
464:
408:
83:
4915:
2023:
1971:
1099:
690:
within the gas through the application of an electric field.
338:
256:
187:
510:. From this fact it is apparent that most of the space of a
138:, so cations and anions attract each other and readily form
4381:
1744:
1483:
743:
the sign; that is, a doubly charged cation is indicated as
724:
458:
402:
222:, meaning "down") and an anion is something that moves up (
210:), meaning "to go". A cation is something that moves down (
206:
was coined from neuter present participle of Greek ἰέναι (
166:
Ions consisting of only a single atom are termed atomic or
92:
68:
900:, but the more usual process of ionization encountered in
74:
51:(F). Forming an ionic bond, Li and F become Li and F ions.
3064:
1715:
1128:). The 3-dimensional shell represents a single arbitrary
449:
393:
274:
as ions that are attracted to the respective electrodes.
3023:
3311:
3252:
600:
by disrupting microbes, and in household items such as
344:
27:
Particle, atom or molecule with a net electrical charge
1054:
991:
925:
596:
As reactive charged particles, they are also used in
473:
455:
446:
417:
399:
396:
77:
3019:
1198:
around the nitrogen atom, making it a very reactive
452:
443:
390:
65:
1088:
1025:
959:
789:Mixed Roman numerals and charge notations for the
727:atom (Fe) that lost two electrons, referred to as
644:
565:Ions can be non-chemically prepared using various
3376:
3167:Goetz, Philip W. (1992). McHenry, Charles (ed.).
182:in liquids, or by other means, such as passing a
170:, while two or more atoms form molecular ions or
4973:
2940:) CS1 maint: bot: original URL status unknown (
2881:) CS1 maint: bot: original URL status unknown (
2822:) CS1 maint: bot: original URL status unknown (
797:Monatomic ions are sometimes also denoted with
876:
4494:
3515:
3444:Chemical elements listed by ionization energy
3132:Harris, William; Levey, Judith, eds. (1976).
714:
186:through a conducting solution, dissolving an
3437:
1275:is much lower than the ionization energy of
1089:{\displaystyle {\ce {Na+ + Cl- -> NaCl}}}
982:, becoming a chloride anion in the process:
633:Inorganic dissolved ions are a component of
3406:
3131:
1045:, NaCl, more commonly known as table salt.
461:
405:
71:
4501:
4487:
3522:
3508:
2919:. Archived from the original on 2021-10-06
2860:. Archived from the original on 2021-10-06
2801:. Archived from the original on 2013-12-24
1140:, in neutral molecules. For example, when
1100:Formation of polyatomic and molecular ions
782:shown in the figure, are thus equivalent.
491:and an ion with a +2 charge is known as a
245:in 1834 for the then-unknown species that
4508:
3529:
3400:
3223:
3015:
3013:
2960:"What Is an Ion? Definition and Examples"
1026:{\displaystyle {\ce {Cl + e- -> Cl-}}}
960:{\displaystyle {\ce {Na -> Na+ + e-}}}
896:Atoms can be ionized by bombardment with
526:
502:Cations and anions are measured by their
3125:
1103:
978:an extra electron and attain a stable 8-
784:
718:
656:
648:
343:
38:
3287:"Atoms and Elements, Isotopes and Ions"
3160:
2983:
2981:
2979:
1212:
197:
14:
4974:
3407:Press, Frank; Siever, Raymond (1986).
3217:
3171:. Vol. 1 (15 ed.). Chicago:
3010:
2936:: CS1 maint: archived copy as title (
2877:: CS1 maint: archived copy as title (
2818:: CS1 maint: archived copy as title (
1305:. The resulting compound is called an
1250:electrons have already been detached.
536:
4482:
3503:
3463:
3461:
3459:
3293:from the original on February 2, 2015
3166:
2987:
2762:Stopping power of radiation particles
1309:, and is said to be held together by
560:
527:introduced by Michael Faraday in 1834
332:
4437:
2976:
2954:
2952:
4461:
3317:"Oxford Reference: OVERVIEW cation"
3104:"The Nobel Prize in Chemistry 1903"
2990:Radiation Detection and Measurement
1317:of individual ions may be derived.
241:) by English physicist and chemist
24:
3456:
3388:from the original on July 13, 2011
3258:"Oxford Reference: OVERVIEW anion"
3226:Dictionary of Scientific Biography
3020:Frank A. J. L. James, ed. (1991).
288:
148:+2 and -2 charge look like this: O
25:
5003:
2949:
701:both use a phenomenon known as a
4569:
4460:
4448:
4436:
4425:
4424:
3224:Cillispie, Charles, ed. (1970).
1282:
529:following his consultation with
439:
386:
61:
3485:from the original on 2018-02-18
3469:"Common Ions and Their Charges"
3370:
3359:from the original on 2011-12-04
3334:
3323:from the original on 2017-01-18
3305:
3275:
3264:from the original on 2017-01-18
3246:
3169:The New Encyclopædia Britannica
3138:(4th ed.). New York City:
3114:from the original on 2018-07-08
3085:from the original on 2011-05-14
3040:from the original on 2021-04-14
645:Detection of ionizing radiation
329:than the parent hydrogen atom.
3283:University of Colorado Boulder
3096:
3071:
3051:
2903:Merriam-Webster.com Dictionary
2890:
2844:Merriam-Webster.com Dictionary
2831:
2775:
1353:
1079:
1009:
930:
831:
637:, a widely known indicator of
579:optical emission spectrometers
13:
1:
3789:Interface and colloid science
3543:Glossary of chemical formulae
3228:(1 ed.). New York City:
3173:Encyclopædia Britannica, Inc.
3135:The New Columbia Encyclopedia
3079:"Online etymology dictionary"
2768:
1243:th electron after the first
871:
723:Equivalent notations for an
709:
480:, from the Greek word κάτω (
7:
4066:Bioorganometallic chemistry
3553:List of inorganic compounds
3411:(14th ed.). New York:
3343:University of South Alabama
3341:Douglas W. Haywick, Ph.D.;
3060:Michael Faraday (1791–1867)
2737:Gaseous ionization detector
2714:
877:Formation of monatomic ions
862:(if positively charged) or
424:, from the Greek word ἄνω (
351:(center) contains a single
10:
5008:
3992:Dynamic covalent chemistry
3963:Enantioselective synthesis
3943:Physical organic chemistry
3896:Organolanthanide chemistry
2992:(3rd ed.). New York:
1830:
1360:
1286:
1216:
715:Denoting the charged state
336:
122:(potassium ion)) while an
43:Electron transfer between
29:
4908:
4880:
4702:
4659:Metal–air electrochemical
4578:
4567:
4516:
4420:
4323:
4084:
4000:
3921:
3871:
3747:
3690:
3581:Electroanalytical methods
3566:
3538:
3413:W. H. Freeman and Company
2628:Anions from organic acids
2626:
2088:
2012:
1848:
1757:
1378:
906:electronic configurations
868:(if negatively charged).
569:, usually involving high
227:
215:
4336:Nobel Prize in Chemistry
4252:Supramolecular chemistry
3891:Organometallic chemistry
2988:Knoll, Glenn F. (1999).
1326:electronic configuration
1177:electronic configuration
4274:Combinatorial chemistry
4185:Food physical chemistry
4148:Environmental chemistry
4032:Bioorthogonal chemistry
3958:Retrosynthetic analysis
3779:Chemical thermodynamics
3762:Spectroelectrochemistry
3705:Computational chemistry
3319:. oxfordreference.com.
3313:Oxford University Press
3260:. oxfordreference.com.
3254:Oxford University Press
3230:Charles Scribner's Sons
1110:electrostatic potential
755:cation is indicated as
541:Ions are ubiquitous in
156:) He (positive charge,
4961:Semipermeable membrane
4750:Lithium–iron–phosphate
4346:of element discoveries
4192:Agricultural chemistry
4180:Carbohydrate chemistry
4071:Bioinorganic chemistry
3936:Alkane stereochemistry
3881:Coordination chemistry
3710:Mathematical chemistry
3576:Instrumental chemistry
2316:Monohydrogen phosphate
1336:electron configuration
1133:
1090:
1027:
980:electron configuration
961:
794:
732:
663:
654:
635:total dissolved solids
368:
52:
4832:Rechargeable alkaline
4510:Electrochemical cells
4341:Timeline of chemistry
4238:Post-mortem chemistry
4223:Clandestine chemistry
4153:Atmospheric chemistry
4076:Biophysical chemistry
3908:Solid-state chemistry
3858:Equilibrium chemistry
3767:Photoelectrochemistry
3380:(November 21, 2013).
3347:"Elemental Chemistry"
3285:(November 21, 2013).
2788:CollinsDictionary.com
1107:
1091:
1028:
970:On the other hand, a
962:
788:
722:
660:
652:
583:particle accelerators
347:
42:
4812:Nickel–metal hydride
4331:History of chemistry
4286:Chemical engineering
4061:Bioorganic chemistry
3811:Structural chemistry
3548:List of biomolecules
3232:. pp. 296–302.
2193:Dihydrogen phosphate
1229:ionization potential
1219:Ionization potential
1213:Ionization potential
1151:, accepts a proton,
1052:
1041:and combine to form
989:
923:
699:proportional counter
198:History of discovery
32:Ion (disambiguation)
30:For other uses, see
4822:Polysulfide–bromide
4664:Nickel oxyhydroxide
4556:Thermogalvanic cell
4354:The central science
4308:Ceramic engineering
4233:Forensic toxicology
4206:Chemistry education
4104:Radiation chemistry
4086:Interdisciplinarity
4039:Medicinal chemistry
3977:Fullerene chemistry
3853:Microwave chemistry
3722:Molecular mechanics
3717:Molecular modelling
3181:1991neb..book.....G
3140:Columbia University
1834:
1364:
836:If an ion contains
805:; for example, the
537:Natural occurrences
136:electrostatic force
130:(chloride ion) and
103:. The charge of an
4987:Physical chemistry
4585:(non-rechargeable)
4529:Concentration cell
4397:Chemical substance
4259:Chemical synthesis
4228:Forensic chemistry
4109:Actinide chemistry
4051:Clinical chemistry
3732:Molecular geometry
3727:Molecular dynamics
3682:Elemental analysis
3635:Separation process
3449:2009-03-30 at the
2757:Ionizing radiation
2606:Aluminium silicate
2219:Hydrogen carbonate
1832:
1362:
1155:—a process called
1134:
1086:
1023:
957:
838:unpaired electrons
801:, particularly in
795:
733:
703:Townsend avalanche
695:Geiger–Müller tube
684:ionization chamber
664:
655:
575:mass spectrometers
561:Related technology
369:
333:Anions and cations
152:(negative charge,
53:
4969:
4968:
4476:
4475:
4412:Quantum mechanics
4377:Chemical compound
4360:Chemical reaction
4298:Materials science
4216:General chemistry
4211:Amateur chemistry
4139:Photogeochemistry
4124:Stellar chemistry
4094:Nuclear chemistry
4015:Molecular biology
3982:Polymer chemistry
3953:Organic synthesis
3948:Organic reactions
3913:Ceramic chemistry
3903:Cluster chemistry
3833:Chemical kinetics
3821:Molecular physics
3700:Quantum chemistry
3613:Mass spectrometry
3378:Purdue University
3239:978-0-684-10112-5
3190:978-0-85229-553-3
3153:978-0-231-03572-9
3003:978-0-471-07338-3
2906:. Merriam-Webster
2847:. Merriam-Webster
2712:
2711:
2708:
2707:
1828:
1827:
1341:electronegativity
1331:electropositivity
1233:ionization energy
1084:
1072:
1059:
1015:
1002:
995:
949:
936:
929:
840:, it is called a
735:When writing the
688:direct ionization
101:electrical charge
16:(Redirected from
4999:
4765:Lithium–titanate
4710:
4586:
4573:
4534:Electric battery
4503:
4496:
4489:
4480:
4479:
4464:
4463:
4452:
4440:
4439:
4428:
4427:
4372:Chemical element
4027:Chemical biology
3886:Magnetochemistry
3863:Mechanochemistry
3816:Chemical physics
3757:Electrochemistry
3662:Characterization
3524:
3517:
3510:
3501:
3500:
3494:
3493:
3491:
3490:
3484:
3473:
3465:
3454:
3441:
3435:
3434:
3404:
3398:
3397:
3395:
3393:
3374:
3368:
3367:
3365:
3364:
3358:
3352:. usouthal.edu.
3351:
3338:
3332:
3331:
3329:
3328:
3309:
3303:
3302:
3300:
3298:
3289:. colorado.edu.
3279:
3273:
3272:
3270:
3269:
3250:
3244:
3243:
3221:
3215:
3214:
3208:
3204:
3202:
3194:
3164:
3158:
3157:
3129:
3123:
3122:
3120:
3119:
3100:
3094:
3093:
3091:
3090:
3075:
3069:
3068:
3055:
3049:
3048:
3046:
3045:
3017:
3008:
3007:
2985:
2974:
2973:
2971:
2970:
2956:
2947:
2945:
2935:
2927:
2925:
2924:
2914:
2912:
2911:
2894:
2888:
2886:
2876:
2868:
2866:
2865:
2855:
2853:
2852:
2835:
2829:
2827:
2817:
2809:
2807:
2806:
2796:
2779:
2702:
2687:
2686:
2685:
2682:
2660:
2645:
2620:
2619:
2618:
2615:
2598:
2597:
2596:
2593:
2576:
2575:
2574:
2571:
2554:
2553:
2552:
2549:
2528:
2527:
2526:
2523:
2506:
2505:
2504:
2501:
2484:
2483:
2482:
2479:
2462:
2461:
2460:
2457:
2440:
2439:
2438:
2435:
2418:
2417:
2416:
2413:
2396:
2395:
2394:
2391:
2374:
2373:
2372:
2369:
2352:
2351:
2350:
2347:
2330:
2329:
2328:
2325:
2308:
2294:
2279:
2278:
2277:
2274:
2265:Hydrogen sulfite
2256:
2255:
2254:
2251:
2242:Hydrogen sulfate
2233:
2232:
2231:
2228:
2211:
2210:
2209:
2206:
2185:
2184:
2183:
2180:
2159:
2158:
2157:
2154:
2137:
2136:
2135:
2132:
2115:
2114:
2113:
2110:
2082:
2081:
2080:
2077:
2060:
2059:
2058:
2055:
2038:
2037:
2036:
2033:
2006:
1992:
1978:
1964:
1950:
1936:
1922:
1908:
1894:
1880:
1866:
1835:
1831:
1821:
1820:
1819:
1816:
1801:
1783:
1782:
1781:
1778:
1751:
1736:
1723:
1708:
1693:
1678:
1663:
1648:
1636:
1623:
1610:
1598:
1584:
1569:
1556:
1543:
1530:
1518:
1503:
1490:
1475:
1462:
1450:
1438:
1424:
1410:
1396:
1365:
1361:
1358:
1357:
1298:chemical bonding
1270:
1266:
1260:valence electron
1249:
1208:
1193:
1192:
1191:
1188:
1174:
1173:
1172:
1169:
1154:
1150:
1139:
1127:
1126:
1125:
1122:
1095:
1093:
1092:
1087:
1085:
1082:
1078:
1077:
1070:
1065:
1064:
1057:
1032:
1030:
1029:
1024:
1022:
1021:
1020:
1013:
1008:
1007:
1000:
993:
966:
964:
963:
958:
956:
955:
954:
947:
942:
941:
934:
927:
816:
812:
808:
781:
777:
773:
765:
758:
737:chemical formula
615:, including the
598:air purification
476:
471:
470:
467:
466:
463:
460:
457:
454:
451:
448:
445:
436:
435:
420:
415:
414:
411:
410:
407:
404:
401:
398:
395:
392:
383:
382:
278:Svante Arrhenius
229:
217:
90:
89:
86:
85:
80:
79:
76:
73:
70:
67:
21:
5007:
5006:
5002:
5001:
5000:
4998:
4997:
4996:
4992:Charge carriers
4972:
4971:
4970:
4965:
4904:
4883:
4876:
4797:Nickel–hydrogen
4755:Lithium–polymer
4711:
4708:
4707:
4698:
4587:
4584:
4583:
4574:
4565:
4512:
4507:
4477:
4472:
4416:
4319:
4313:Polymer science
4269:Click chemistry
4264:Green chemistry
4158:Ocean chemistry
4134:Biogeochemistry
4080:
3996:
3968:Total synthesis
3931:Stereochemistry
3917:
3867:
3784:Surface science
3774:Thermochemistry
3743:
3686:
3657:Crystallography
3562:
3534:
3528:
3498:
3497:
3488:
3486:
3482:
3471:
3467:
3466:
3457:
3451:Wayback Machine
3442:
3438:
3423:
3405:
3401:
3391:
3389:
3375:
3371:
3362:
3360:
3356:
3349:
3339:
3335:
3326:
3324:
3310:
3306:
3296:
3294:
3280:
3276:
3267:
3265:
3251:
3247:
3240:
3222:
3218:
3206:
3205:
3196:
3195:
3191:
3165:
3161:
3154:
3130:
3126:
3117:
3115:
3102:
3101:
3097:
3088:
3086:
3077:
3076:
3072:
3057:
3056:
3052:
3043:
3041:
3034:
3026:. p. 183.
3018:
3011:
3004:
2986:
2977:
2968:
2966:
2958:
2957:
2950:
2929:
2928:
2922:
2920:
2917:"Archived copy"
2915:
2909:
2907:
2896:
2895:
2891:
2870:
2869:
2863:
2861:
2858:"Archived copy"
2856:
2850:
2848:
2837:
2836:
2832:
2811:
2810:
2804:
2802:
2799:"Archived copy"
2797:
2781:
2780:
2776:
2771:
2766:
2717:
2700:
2683:
2680:
2679:
2677:
2673:
2658:
2643:
2639:
2616:
2613:
2612:
2610:
2594:
2591:
2590:
2588:
2572:
2569:
2568:
2566:
2550:
2547:
2546:
2544:
2540:
2524:
2521:
2520:
2518:
2502:
2499:
2498:
2496:
2480:
2477:
2476:
2474:
2458:
2455:
2454:
2452:
2436:
2433:
2432:
2430:
2414:
2411:
2410:
2408:
2392:
2389:
2388:
2386:
2370:
2367:
2366:
2364:
2348:
2345:
2344:
2342:
2326:
2323:
2322:
2320:
2306:
2292:
2275:
2272:
2271:
2269:
2252:
2249:
2248:
2246:
2229:
2226:
2225:
2223:
2207:
2204:
2203:
2201:
2197:
2181:
2178:
2177:
2175:
2171:
2155:
2152:
2151:
2149:
2133:
2130:
2129:
2127:
2111:
2108:
2107:
2105:
2092:Polyatomic ions
2078:
2075:
2074:
2072:
2056:
2053:
2052:
2050:
2034:
2031:
2030:
2028:
2004:
1990:
1976:
1962:
1948:
1934:
1920:
1906:
1892:
1878:
1864:
1817:
1814:
1813:
1811:
1799:
1795:
1779:
1776:
1775:
1773:
1749:
1734:
1721:
1706:
1691:
1676:
1661:
1646:
1634:
1621:
1608:
1596:
1582:
1567:
1554:
1541:
1528:
1516:
1501:
1488:
1473:
1460:
1448:
1436:
1422:
1408:
1394:
1363:Common cations
1356:
1303:crystal lattice
1291:
1285:
1268:
1264:
1255:atomic orbitals
1244:
1221:
1215:
1206:
1189:
1186:
1185:
1183:
1170:
1167:
1166:
1164:
1152:
1149:
1145:
1137:
1123:
1120:
1119:
1117:
1102:
1073:
1069:
1060:
1056:
1055:
1053:
1050:
1049:
1043:sodium chloride
1016:
1012:
1003:
999:
992:
990:
987:
986:
950:
946:
937:
933:
926:
924:
921:
920:
911:For example, a
879:
874:
834:
821:oxidation state
814:
810:
806:
779:
775:
771:
763:
756:
717:
712:
647:
602:smoke detectors
563:
539:
531:William Whewell
474:
442:
438:
433:
432:
418:
389:
385:
380:
379:
342:
335:
291:
289:Characteristics
243:Michael Faraday
239:William Whewell
200:
172:polyatomic ions
151:
140:ionic compounds
82:
64:
60:
35:
28:
23:
22:
15:
12:
11:
5:
5005:
4995:
4994:
4989:
4984:
4967:
4966:
4964:
4963:
4958:
4953:
4948:
4943:
4938:
4933:
4928:
4923:
4918:
4912:
4910:
4906:
4905:
4903:
4902:
4897:
4892:
4890:Atomic battery
4886:
4884:
4881:
4878:
4877:
4875:
4874:
4869:
4864:
4862:Vanadium redox
4859:
4854:
4849:
4844:
4839:
4837:Silver–cadmium
4834:
4829:
4824:
4819:
4814:
4809:
4807:Nickel–lithium
4804:
4799:
4794:
4792:Nickel–cadmium
4789:
4784:
4779:
4774:
4769:
4768:
4767:
4762:
4760:Lithium–sulfur
4757:
4752:
4747:
4737:
4732:
4731:
4730:
4720:
4714:
4712:
4709:(rechargeable)
4705:Secondary cell
4703:
4700:
4699:
4697:
4696:
4691:
4686:
4681:
4676:
4671:
4666:
4661:
4656:
4651:
4646:
4641:
4636:
4631:
4629:Edison–Lalande
4626:
4621:
4616:
4611:
4606:
4601:
4596:
4590:
4588:
4579:
4576:
4575:
4568:
4566:
4564:
4563:
4558:
4553:
4548:
4547:
4546:
4544:Trough battery
4541:
4531:
4526:
4520:
4518:
4514:
4513:
4506:
4505:
4498:
4491:
4483:
4474:
4473:
4471:
4470:
4458:
4446:
4434:
4421:
4418:
4417:
4415:
4414:
4409:
4404:
4399:
4394:
4389:
4384:
4379:
4374:
4369:
4368:
4367:
4357:
4350:
4349:
4348:
4338:
4333:
4327:
4325:
4321:
4320:
4318:
4317:
4316:
4315:
4310:
4305:
4295:
4294:
4293:
4283:
4282:
4281:
4276:
4271:
4266:
4256:
4255:
4254:
4243:
4242:
4241:
4240:
4235:
4225:
4220:
4219:
4218:
4213:
4202:
4201:
4200:
4199:
4197:Soil chemistry
4189:
4188:
4187:
4182:
4175:Food chemistry
4172:
4170:Carbochemistry
4167:
4165:Clay chemistry
4162:
4161:
4160:
4155:
4144:
4143:
4142:
4141:
4136:
4126:
4120:Astrochemistry
4116:Cosmochemistry
4113:
4112:
4111:
4106:
4101:
4099:Radiochemistry
4090:
4088:
4082:
4081:
4079:
4078:
4073:
4068:
4063:
4058:
4056:Neurochemistry
4053:
4048:
4047:
4046:
4036:
4035:
4034:
4024:
4023:
4022:
4017:
4006:
4004:
3998:
3997:
3995:
3994:
3989:
3987:Petrochemistry
3984:
3979:
3974:
3965:
3960:
3955:
3950:
3945:
3940:
3939:
3938:
3927:
3925:
3919:
3918:
3916:
3915:
3910:
3905:
3900:
3899:
3898:
3888:
3883:
3877:
3875:
3869:
3868:
3866:
3865:
3860:
3855:
3850:
3848:Spin chemistry
3845:
3843:Photochemistry
3840:
3835:
3830:
3828:Femtochemistry
3825:
3824:
3823:
3813:
3808:
3803:
3798:
3797:
3796:
3786:
3781:
3776:
3771:
3770:
3769:
3764:
3753:
3751:
3745:
3744:
3742:
3741:
3740:
3739:
3729:
3724:
3719:
3714:
3713:
3712:
3702:
3696:
3694:
3688:
3687:
3685:
3684:
3679:
3674:
3669:
3664:
3659:
3654:
3653:
3652:
3647:
3640:Chromatography
3637:
3632:
3631:
3630:
3625:
3620:
3610:
3609:
3608:
3603:
3598:
3593:
3583:
3578:
3572:
3570:
3564:
3563:
3561:
3560:
3558:Periodic table
3555:
3550:
3545:
3539:
3536:
3535:
3527:
3526:
3519:
3512:
3504:
3496:
3495:
3455:
3453:. Lenntech.com
3436:
3421:
3415:. p. 63.
3399:
3384:. purdue.edu.
3369:
3333:
3304:
3274:
3245:
3238:
3216:
3207:|journal=
3189:
3159:
3152:
3124:
3108:nobelprize.org
3095:
3070:
3050:
3032:
3009:
3002:
2975:
2948:
2889:
2830:
2773:
2772:
2770:
2767:
2765:
2764:
2759:
2754:
2749:
2744:
2739:
2734:
2729:
2724:
2718:
2716:
2713:
2710:
2709:
2706:
2705:
2703:
2698:
2692:
2691:
2688:
2675:
2671:
2665:
2664:
2661:
2656:
2650:
2649:
2646:
2641:
2637:
2631:
2630:
2624:
2623:
2621:
2608:
2602:
2601:
2599:
2586:
2580:
2579:
2577:
2564:
2558:
2557:
2555:
2542:
2538:
2532:
2531:
2529:
2516:
2510:
2509:
2507:
2494:
2488:
2487:
2485:
2472:
2466:
2465:
2463:
2450:
2444:
2443:
2441:
2428:
2422:
2421:
2419:
2406:
2400:
2399:
2397:
2384:
2378:
2377:
2375:
2362:
2356:
2355:
2353:
2340:
2334:
2333:
2331:
2318:
2312:
2311:
2309:
2304:
2298:
2297:
2295:
2290:
2284:
2283:
2280:
2267:
2261:
2260:
2257:
2244:
2238:
2237:
2234:
2221:
2215:
2214:
2212:
2199:
2195:
2189:
2188:
2186:
2173:
2169:
2163:
2162:
2160:
2147:
2141:
2140:
2138:
2125:
2119:
2118:
2116:
2103:
2097:
2096:
2086:
2085:
2083:
2070:
2064:
2063:
2061:
2048:
2042:
2041:
2039:
2026:
2020:
2019:
2010:
2009:
2007:
2002:
1996:
1995:
1993:
1988:
1982:
1981:
1979:
1974:
1968:
1967:
1965:
1960:
1954:
1953:
1951:
1946:
1940:
1939:
1937:
1932:
1926:
1925:
1923:
1918:
1912:
1911:
1909:
1904:
1898:
1897:
1895:
1890:
1884:
1883:
1881:
1876:
1870:
1869:
1867:
1862:
1856:
1855:
1846:
1845:
1842:
1839:
1833:Common anions
1829:
1826:
1825:
1822:
1809:
1805:
1804:
1802:
1797:
1793:
1787:
1786:
1784:
1771:
1765:
1764:
1755:
1754:
1752:
1747:
1741:
1740:
1737:
1732:
1728:
1727:
1724:
1719:
1712:
1711:
1709:
1704:
1698:
1697:
1694:
1689:
1683:
1682:
1679:
1674:
1668:
1667:
1664:
1659:
1653:
1652:
1649:
1644:
1640:
1639:
1637:
1632:
1628:
1627:
1624:
1619:
1618:Manganese(III)
1615:
1614:
1611:
1606:
1602:
1601:
1599:
1594:
1588:
1587:
1585:
1580:
1574:
1573:
1570:
1565:
1561:
1560:
1557:
1552:
1548:
1547:
1544:
1539:
1535:
1534:
1531:
1526:
1522:
1521:
1519:
1514:
1508:
1507:
1504:
1499:
1495:
1494:
1491:
1486:
1480:
1479:
1476:
1471:
1467:
1466:
1463:
1458:
1454:
1453:
1451:
1446:
1442:
1441:
1439:
1434:
1428:
1427:
1425:
1420:
1414:
1413:
1411:
1406:
1400:
1399:
1397:
1392:
1386:
1385:
1376:
1375:
1374:Historic name
1372:
1369:
1355:
1352:
1307:ionic compound
1287:Main article:
1284:
1281:
1217:Main article:
1214:
1211:
1159:—it forms the
1147:
1101:
1098:
1097:
1096:
1081:
1076:
1068:
1063:
1034:
1033:
1019:
1011:
1006:
998:
968:
967:
953:
945:
940:
932:
887:atomic nucleus
878:
875:
873:
870:
833:
830:
799:Roman numerals
716:
713:
711:
708:
646:
643:
587:ion implanters
562:
559:
538:
535:
361:hydrogen anion
334:
331:
323:electron cloud
312:magnetic field
290:
287:
199:
196:
184:direct current
168:monatomic ions
149:
26:
9:
6:
4:
3:
2:
5004:
4993:
4990:
4988:
4985:
4983:
4980:
4979:
4977:
4962:
4959:
4957:
4954:
4952:
4949:
4947:
4944:
4942:
4939:
4937:
4934:
4932:
4929:
4927:
4924:
4922:
4919:
4917:
4914:
4913:
4911:
4907:
4901:
4898:
4896:
4893:
4891:
4888:
4887:
4885:
4879:
4873:
4870:
4868:
4865:
4863:
4860:
4858:
4855:
4853:
4852:Sodium–sulfur
4850:
4848:
4845:
4843:
4840:
4838:
4835:
4833:
4830:
4828:
4827:Potassium ion
4825:
4823:
4820:
4818:
4815:
4813:
4810:
4808:
4805:
4803:
4800:
4798:
4795:
4793:
4790:
4788:
4785:
4783:
4780:
4778:
4775:
4773:
4770:
4766:
4763:
4761:
4758:
4756:
4753:
4751:
4748:
4746:
4743:
4742:
4741:
4738:
4736:
4733:
4729:
4726:
4725:
4724:
4721:
4719:
4716:
4715:
4713:
4706:
4701:
4695:
4692:
4690:
4687:
4685:
4682:
4680:
4677:
4675:
4672:
4670:
4667:
4665:
4662:
4660:
4657:
4655:
4652:
4650:
4647:
4645:
4644:Lithium metal
4642:
4640:
4637:
4635:
4632:
4630:
4627:
4625:
4622:
4620:
4617:
4615:
4612:
4610:
4607:
4605:
4602:
4600:
4599:Aluminium–air
4597:
4595:
4592:
4591:
4589:
4582:
4577:
4572:
4562:
4559:
4557:
4554:
4552:
4549:
4545:
4542:
4540:
4537:
4536:
4535:
4532:
4530:
4527:
4525:
4524:Galvanic cell
4522:
4521:
4519:
4515:
4511:
4504:
4499:
4497:
4492:
4490:
4485:
4484:
4481:
4469:
4468:
4459:
4457:
4456:
4451:
4447:
4445:
4444:
4435:
4433:
4432:
4423:
4422:
4419:
4413:
4410:
4408:
4405:
4403:
4402:Chemical bond
4400:
4398:
4395:
4393:
4390:
4388:
4385:
4383:
4380:
4378:
4375:
4373:
4370:
4366:
4363:
4362:
4361:
4358:
4355:
4351:
4347:
4344:
4343:
4342:
4339:
4337:
4334:
4332:
4329:
4328:
4326:
4322:
4314:
4311:
4309:
4306:
4304:
4301:
4300:
4299:
4296:
4292:
4291:Stoichiometry
4289:
4288:
4287:
4284:
4280:
4277:
4275:
4272:
4270:
4267:
4265:
4262:
4261:
4260:
4257:
4253:
4250:
4249:
4248:
4247:Nanochemistry
4245:
4244:
4239:
4236:
4234:
4231:
4230:
4229:
4226:
4224:
4221:
4217:
4214:
4212:
4209:
4208:
4207:
4204:
4203:
4198:
4195:
4194:
4193:
4190:
4186:
4183:
4181:
4178:
4177:
4176:
4173:
4171:
4168:
4166:
4163:
4159:
4156:
4154:
4151:
4150:
4149:
4146:
4145:
4140:
4137:
4135:
4132:
4131:
4130:
4127:
4125:
4121:
4117:
4114:
4110:
4107:
4105:
4102:
4100:
4097:
4096:
4095:
4092:
4091:
4089:
4087:
4083:
4077:
4074:
4072:
4069:
4067:
4064:
4062:
4059:
4057:
4054:
4052:
4049:
4045:
4042:
4041:
4040:
4037:
4033:
4030:
4029:
4028:
4025:
4021:
4018:
4016:
4013:
4012:
4011:
4008:
4007:
4005:
4003:
3999:
3993:
3990:
3988:
3985:
3983:
3980:
3978:
3975:
3973:
3972:Semisynthesis
3969:
3966:
3964:
3961:
3959:
3956:
3954:
3951:
3949:
3946:
3944:
3941:
3937:
3934:
3933:
3932:
3929:
3928:
3926:
3924:
3920:
3914:
3911:
3909:
3906:
3904:
3901:
3897:
3894:
3893:
3892:
3889:
3887:
3884:
3882:
3879:
3878:
3876:
3874:
3870:
3864:
3861:
3859:
3856:
3854:
3851:
3849:
3846:
3844:
3841:
3839:
3836:
3834:
3831:
3829:
3826:
3822:
3819:
3818:
3817:
3814:
3812:
3809:
3807:
3806:Sonochemistry
3804:
3802:
3801:Cryochemistry
3799:
3795:
3794:Micromeritics
3792:
3791:
3790:
3787:
3785:
3782:
3780:
3777:
3775:
3772:
3768:
3765:
3763:
3760:
3759:
3758:
3755:
3754:
3752:
3750:
3746:
3738:
3735:
3734:
3733:
3730:
3728:
3725:
3723:
3720:
3718:
3715:
3711:
3708:
3707:
3706:
3703:
3701:
3698:
3697:
3695:
3693:
3689:
3683:
3680:
3678:
3675:
3673:
3672:Wet chemistry
3670:
3668:
3665:
3663:
3660:
3658:
3655:
3651:
3648:
3646:
3643:
3642:
3641:
3638:
3636:
3633:
3629:
3626:
3624:
3621:
3619:
3616:
3615:
3614:
3611:
3607:
3604:
3602:
3599:
3597:
3594:
3592:
3589:
3588:
3587:
3584:
3582:
3579:
3577:
3574:
3573:
3571:
3569:
3565:
3559:
3556:
3554:
3551:
3549:
3546:
3544:
3541:
3540:
3537:
3533:
3525:
3520:
3518:
3513:
3511:
3506:
3505:
3502:
3481:
3477:
3470:
3464:
3462:
3460:
3452:
3448:
3445:
3440:
3432:
3428:
3424:
3422:0-7167-1743-3
3418:
3414:
3410:
3403:
3387:
3383:
3382:"Amino Acids"
3379:
3373:
3355:
3348:
3345:(2007–2008).
3344:
3337:
3322:
3318:
3314:
3308:
3292:
3288:
3284:
3278:
3263:
3259:
3255:
3249:
3241:
3235:
3231:
3227:
3220:
3212:
3200:
3192:
3186:
3182:
3178:
3175:p. 587.
3174:
3170:
3163:
3155:
3149:
3145:
3141:
3137:
3136:
3128:
3113:
3109:
3105:
3099:
3084:
3080:
3074:
3066:
3062:
3061:
3054:
3039:
3035:
3033:9780863412493
3029:
3025:
3024:
3016:
3014:
3005:
2999:
2995:
2991:
2984:
2982:
2980:
2965:
2961:
2955:
2953:
2943:
2939:
2933:
2918:
2905:
2904:
2899:
2893:
2884:
2880:
2874:
2859:
2846:
2845:
2840:
2834:
2825:
2821:
2815:
2800:
2794:
2793:HarperCollins
2790:
2789:
2784:
2778:
2774:
2763:
2760:
2758:
2755:
2753:
2750:
2748:
2745:
2743:
2740:
2738:
2735:
2733:
2730:
2728:
2725:
2723:
2720:
2719:
2704:
2699:
2697:
2694:
2693:
2690:ethanedioate
2689:
2672:
2670:
2667:
2666:
2662:
2657:
2655:
2652:
2651:
2647:
2638:
2636:
2633:
2632:
2629:
2625:
2622:
2609:
2607:
2604:
2603:
2600:
2587:
2585:
2582:
2581:
2578:
2565:
2563:
2560:
2559:
2556:
2539:
2537:
2534:
2533:
2530:
2517:
2515:
2512:
2511:
2508:
2495:
2493:
2490:
2489:
2486:
2473:
2471:
2468:
2467:
2464:
2451:
2449:
2446:
2445:
2442:
2429:
2427:
2424:
2423:
2420:
2407:
2405:
2402:
2401:
2398:
2385:
2383:
2380:
2379:
2376:
2363:
2361:
2358:
2357:
2354:
2341:
2339:
2336:
2335:
2332:
2319:
2317:
2314:
2313:
2310:
2305:
2303:
2300:
2299:
2296:
2291:
2289:
2286:
2285:
2281:
2268:
2266:
2263:
2262:
2258:
2245:
2243:
2240:
2239:
2235:
2222:
2220:
2217:
2216:
2213:
2196:
2194:
2191:
2190:
2187:
2170:
2168:
2165:
2164:
2161:
2148:
2146:
2143:
2142:
2139:
2126:
2124:
2121:
2120:
2117:
2104:
2102:
2099:
2098:
2095:
2093:
2087:
2084:
2071:
2069:
2066:
2065:
2062:
2049:
2047:
2044:
2043:
2040:
2027:
2025:
2022:
2021:
2018:
2016:
2011:
2008:
2003:
2001:
1998:
1997:
1994:
1989:
1987:
1984:
1983:
1980:
1975:
1973:
1970:
1969:
1966:
1961:
1959:
1956:
1955:
1952:
1947:
1945:
1942:
1941:
1938:
1933:
1931:
1928:
1927:
1924:
1919:
1917:
1914:
1913:
1910:
1905:
1903:
1900:
1899:
1896:
1891:
1889:
1886:
1885:
1882:
1877:
1875:
1872:
1871:
1868:
1863:
1861:
1858:
1857:
1854:
1852:
1847:
1843:
1840:
1837:
1836:
1823:
1810:
1807:
1806:
1803:
1794:
1792:
1789:
1788:
1785:
1772:
1770:
1767:
1766:
1763:
1761:
1756:
1753:
1748:
1746:
1743:
1742:
1738:
1733:
1730:
1729:
1725:
1720:
1717:
1714:
1713:
1710:
1705:
1703:
1700:
1699:
1695:
1690:
1688:
1685:
1684:
1680:
1675:
1673:
1670:
1669:
1665:
1660:
1658:
1655:
1654:
1650:
1645:
1642:
1641:
1638:
1633:
1631:Manganese(IV)
1630:
1629:
1625:
1620:
1617:
1616:
1612:
1607:
1605:Manganese(II)
1604:
1603:
1600:
1595:
1593:
1590:
1589:
1586:
1581:
1579:
1576:
1575:
1571:
1566:
1563:
1562:
1558:
1553:
1550:
1549:
1545:
1540:
1537:
1536:
1532:
1527:
1524:
1523:
1520:
1515:
1513:
1510:
1509:
1505:
1500:
1497:
1496:
1492:
1487:
1485:
1482:
1481:
1477:
1472:
1469:
1468:
1464:
1459:
1456:
1455:
1452:
1447:
1445:Chromium(III)
1444:
1443:
1440:
1435:
1433:
1430:
1429:
1426:
1421:
1419:
1416:
1415:
1412:
1407:
1405:
1402:
1401:
1398:
1393:
1391:
1388:
1387:
1384:
1382:
1377:
1373:
1370:
1367:
1366:
1359:
1351:
1349:
1348:
1343:
1342:
1337:
1333:
1332:
1327:
1323:
1318:
1316:
1312:
1311:ionic bonding
1308:
1304:
1299:
1296:is a kind of
1295:
1294:Ionic bonding
1290:
1283:Ionic bonding
1280:
1278:
1274:
1262:
1261:
1256:
1251:
1247:
1242:
1238:
1234:
1230:
1226:
1220:
1210:
1203:
1201:
1197:
1196:valence shell
1180:
1178:
1162:
1158:
1143:
1131:
1115:
1111:
1106:
1074:
1066:
1061:
1048:
1047:
1046:
1044:
1040:
1017:
1004:
996:
985:
984:
983:
981:
977:
973:
951:
943:
938:
919:
918:
917:
914:
909:
907:
903:
899:
894:
892:
888:
884:
883:valence shell
869:
867:
866:
861:
860:
855:
851:
850:
845:
844:
839:
829:
827:
823:
822:
804:
800:
792:
787:
783:
767:
762:
754:
750:
746:
742:
738:
730:
726:
721:
707:
704:
700:
696:
691:
689:
685:
681:
677:
673:
669:
659:
651:
642:
640:
639:water quality
636:
631:
629:
625:
621:
618:
614:
610:
605:
603:
599:
594:
592:
588:
584:
580:
576:
572:
568:
558:
556:
552:
548:
544:
534:
532:
528:
524:
520:
515:
513:
509:
505:
500:
498:
494:
490:
485:
483:
479:
478:
469:
429:
427:
423:
422:
413:
376:
373:
366:
362:
358:
355:and a single
354:
350:
349:Hydrogen atom
346:
340:
330:
328:
324:
320:
315:
313:
308:
305:
301:
297:
296:solvated ions
286:
283:
279:
275:
273:
269:
266:, as well as
265:
264:
259:
258:
252:
248:
244:
240:
237:
233:
225:
221:
213:
209:
205:
195:
193:
189:
185:
181:
177:
173:
169:
164:
162:
159:
155:
146:
143:
141:
137:
133:
129:
125:
121:
117:
112:
110:
106:
102:
98:
94:
88:
58:
50:
46:
41:
37:
33:
19:
4950:
4867:Zinc–bromine
4674:Silver oxide
4609:Chromic acid
4581:Primary cell
4561:Voltaic pile
4539:Flow battery
4465:
4453:
4441:
4429:
4391:
4279:Biosynthesis
4129:Geochemistry
4044:Pharmacology
4020:Cell biology
4010:Biochemistry
3838:Spectroscopy
3737:VSEPR theory
3586:Spectroscopy
3530:Branches of
3487:. Retrieved
3476:Science Geek
3475:
3439:
3408:
3402:
3392:November 22,
3390:. Retrieved
3372:
3361:. Retrieved
3336:
3325:. Retrieved
3307:
3297:November 22,
3295:. Retrieved
3277:
3266:. Retrieved
3248:
3225:
3219:
3168:
3162:
3134:
3127:
3116:. Retrieved
3107:
3098:
3087:. Retrieved
3073:
3059:
3053:
3042:. Retrieved
3022:
2989:
2967:. Retrieved
2963:
2921:. Retrieved
2908:. Retrieved
2901:
2892:
2862:. Retrieved
2849:. Retrieved
2842:
2833:
2803:. Retrieved
2786:
2777:
2752:Ion exchange
2627:
2584:Metasilicate
2404:Permanganate
2302:Hypochlorite
2236:bicarbonate
2089:
2013:
1849:
1838:Formal name
1758:
1379:
1368:Common name
1345:
1339:
1329:
1319:
1315:ionic radius
1310:
1306:
1293:
1292:
1258:
1252:
1245:
1240:
1236:
1232:
1228:
1222:
1204:
1181:
1135:
1130:isopotential
1035:
975:
969:
910:
895:
890:
880:
863:
857:
854:organic ions
853:
847:
841:
835:
825:
818:
803:spectroscopy
796:
768:
760:
748:
744:
740:
734:
692:
687:
665:
632:
624:amphotericin
617:ion channels
606:
595:
564:
540:
522:
518:
516:
504:ionic radius
501:
486:
481:
430:
425:
377:
374:
370:
327:much smaller
326:
319:matter waves
316:
309:
295:
292:
276:
271:
267:
261:
255:
246:
231:
219:
207:
203:
201:
165:
147:
144:
123:
115:
113:
56:
54:
36:
4956:Salt bridge
4941:Electrolyte
4872:Zinc–cerium
4857:Solid state
4842:Silver–zinc
4817:Nickel–zinc
4802:Nickel–iron
4777:Molten salt
4745:Dual carbon
4740:Lithium ion
4735:Lithium–air
4694:Zinc–carbon
4669:Silicon–air
4649:Lithium–air
4467:WikiProject
3692:Theoretical
3677:Calorimetry
2732:Electrolyte
2722:Air ioniser
2663:methanoate
2536:Thiosulfate
2382:Perchlorate
2090:Oxoanions (
1643:Mercury(II)
1354:Common ions
1322:noble gases
1157:protonation
1114:nitrate ion
1112:map of the
1039:ionic bonds
859:carbocation
832:Sub-classes
747:instead of
591:ion engines
567:ion sources
176:dissolution
99:with a net
4976:Categories
4909:Cell parts
4900:Solar cell
4882:Other cell
4847:Sodium ion
4718:Automotive
4303:Metallurgy
4002:Biological
3568:Analytical
3489:2018-05-11
3363:2013-11-22
3327:2017-01-15
3268:2017-01-15
3142:. p.
3118:2017-06-13
3089:2011-01-07
3044:2020-10-16
2969:2024-08-26
2923:2021-10-06
2910:2021-10-06
2864:2021-10-06
2851:2021-10-06
2805:2013-12-21
2769:References
2648:ethanoate
2514:Superoxide
2282:bisulfite
2259:bisulfate
2167:Dichromate
2015:Polyatomic
1844:Alt. name
1824:mercurous
1808:Mercury(I)
1760:Polyatomic
1681:argentous
1613:manganous
1470:Copper(II)
1289:Ionic bond
891:ionization
620:gramicidin
547:ionosphere
517:The terms
497:zwitterion
282:dissociate
192:ionization
4946:Half-cell
4936:Electrode
4895:Fuel cell
4772:Metal–air
4723:Lead–acid
4639:Leclanché
4551:Fuel cell
4365:Catalysis
3873:Inorganic
3667:Titration
3532:chemistry
3209:ignored (
3199:cite book
2964:ThoughtCo
2742:Ioliomics
2448:Phosphate
2288:Hydroxide
2101:Carbonate
1958:Phosphide
1851:Monatomic
1791:Hydronium
1726:stannous
1702:Strontium
1657:Potassium
1651:mercuric
1626:manganic
1592:Magnesium
1559:plumbous
1538:Iron(III)
1498:Gold(III)
1457:Copper(I)
1418:Beryllium
1390:Aluminium
1381:Monatomic
1277:nonmetals
1080:⟶
1075:−
1018:−
1010:⟶
1005:−
952:−
931:⟶
902:chemistry
898:radiation
872:Formation
865:carbanion
849:oxyanions
710:Chemistry
662:electron.
628:fungicide
609:membranes
551:gemstones
251:electrode
249:from one
202:The word
47:(Li) and
4926:Catalyst
4787:Nanowire
4782:Nanopore
4728:gel–VRLA
4689:Zinc–air
4594:Alkaline
4431:Category
4387:Molecule
4324:See also
3749:Physical
3480:Archived
3447:Archived
3431:12556840
3386:Archived
3354:Archived
3321:Archived
3315:(2013).
3291:Archived
3262:Archived
3256:(2013).
3112:Archived
3083:Archived
3038:Archived
2932:cite web
2873:cite web
2839:"cation"
2814:cite web
2747:Ion beam
2715:See also
2562:Silicate
2426:Peroxide
2145:Chromate
2123:Chlorate
2068:Triodide
2046:Peroxide
2000:Selenide
1902:Fluoride
1888:Chloride
1841:Formula
1769:Ammonium
1739:stannic
1572:plumbic
1564:Lead(IV)
1551:Lead(II)
1533:ferrous
1525:Iron(II)
1465:cuprous
1371:Formula
1161:ammonium
972:chlorine
697:and the
613:biocides
493:dication
357:electron
236:polymath
161:particle
154:peroxide
105:electron
97:molecule
91:) is an
49:fluorine
18:Nonionic
4931:Cathode
4684:Zamboni
4654:Mercury
4619:Daniell
4443:Commons
4407:Alchemy
3923:Organic
3177:Bibcode
2898:"anion"
2696:Cyanide
2669:Oxalate
2654:Formate
2635:Acetate
2492:Sulfite
2470:Sulfate
2360:Nitrite
2338:Nitrate
1986:Sulfide
1944:Nitride
1916:Hydride
1874:Carbide
1860:Bromide
1762:cations
1731:Tin(IV)
1696:natric
1578:Lithium
1546:ferric
1493:aurous
1484:Gold(I)
1478:cupric
1432:Calcium
1383:cations
1200:radical
1142:ammonia
843:radical
819:formal
811:Fe(III)
729:ferrous
571:voltage
512:crystal
489:dianion
477:-eye-ən
421:-eye-ən
304:entropy
263:cathode
45:lithium
4921:Binder
4679:Weston
4604:Bunsen
4455:Portal
3601:UV-Vis
3429:
3419:
3236:
3187:
3150:
3063:. UK:
3030:
3000:
2727:Aurora
2017:anions
1930:Iodide
1853:anions
1687:Sodium
1672:Silver
1666:kalic
1512:Hydron
1506:auric
1404:Barium
1273:metals
1235:. The
1225:energy
913:sodium
826:cannot
791:uranyl
778:, and
753:sodium
741:before
680:X-rays
678:, and
589:, and
543:nature
523:cation
508:oxygen
434:cation
365:cation
353:proton
300:energy
272:cation
116:cation
109:proton
4916:Anode
4634:Grove
4614:Clark
4517:Types
3628:MALDI
3596:Raman
3483:(PDF)
3472:(PDF)
3409:Earth
3357:(PDF)
3350:(PDF)
2994:Wiley
2783:"ion"
2611:AlSiO
2024:Azide
1972:Oxide
1231:, or
1202:ion.
1163:ion,
676:gamma
668:alpha
519:anion
437:(+) (
384:(−) (
381:anion
339:Anyon
268:anion
257:anode
224:Greek
212:Greek
208:ienai
188:anode
178:of a
158:alpha
124:anion
4982:Ions
4951:Ions
4382:Atom
3650:HPLC
3427:OCLC
3417:ISBN
3394:2013
3299:2013
3234:ISBN
3211:help
3185:ISBN
3148:ISBN
3028:ISBN
2998:ISBN
2942:link
2938:link
2883:link
2879:link
2824:link
2820:link
2659:HCOO
1745:Zinc
1718:(II)
1347:salt
1223:The
1083:NaCl
976:gain
759:and
725:iron
693:The
672:beta
622:and
521:and
495:. A
482:kátō
302:and
270:and
260:and
247:goes
220:kato
216:κάτω
190:via
180:salt
93:atom
4624:Dry
4392:Ion
3623:ICP
3606:NMR
3144:155
3065:BBC
2644:COO
2589:SiO
2567:SiO
2409:MnO
2387:ClO
2321:HPO
2307:ClO
2270:HSO
2247:HSO
2224:HCO
2150:CrO
2128:ClO
1716:Tin
1248:− 1
1118:2NO
1108:An
770:of
761:not
630:).
626:(a
555:ATP
475:KAT
426:ánō
419:ANN
378:An
232:ano
228:ἄνω
204:ion
163:).
95:or
57:ion
55:An
4978::
4122:/
4118:/
3970:/
3645:GC
3618:EI
3591:IR
3478:.
3474:.
3458:^
3425:.
3203::
3201:}}
3197:{{
3183:.
3146:.
3110:.
3106:.
3081:.
3036:.
3012:^
2996:.
2978:^
2962:.
2951:^
2934:}}
2930:{{
2900:.
2875:}}
2871:{{
2841:.
2816:}}
2812:{{
2791:.
2785:.
2701:CN
2681:2−
2640:CH
2592:2−
2570:4−
2548:2−
2500:2−
2497:SO
2478:2−
2475:SO
2456:3−
2453:PO
2434:2−
2365:NO
2343:NO
2324:2−
2293:OH
2202:PO
2179:2−
2172:Cr
2153:2−
2109:2−
2106:CO
2054:2−
2005:Se
1893:Cl
1865:Br
1815:2+
1812:Hg
1774:NH
1750:Zn
1735:Sn
1722:Sn
1707:Sr
1692:Na
1677:Ag
1647:Hg
1635:Mn
1622:Mn
1609:Mn
1597:Mg
1583:Li
1568:Pb
1555:Pb
1542:Fe
1529:Fe
1502:Au
1489:Au
1474:Cu
1461:Cu
1449:Cr
1437:Ca
1423:Be
1409:Ba
1395:Al
1350:.
1269:Cl
1265:Na
1184:NH
1165:NH
1146:NH
1144:,
1071:Cl
1058:Na
1014:Cl
994:Cl
935:Na
928:Na
893:.
815:Fe
813:,
807:Fe
780:Fe
776:Fe
774:,
772:Fe
766:.
764:Na
757:Na
749:+2
745:2+
674:,
670:,
641:.
604:.
593:.
585:,
581:,
577:,
533:.
465:ən
459:aɪ
431:A
409:ən
403:aɪ
314:.
230:,
226::
218:,
214::
194:.
142:.
132:OH
128:Cl
114:A
84:ən
81:,-
69:aɪ
4502:e
4495:t
4488:v
4356:"
4352:"
3523:e
3516:t
3509:v
3492:.
3433:.
3396:.
3366:.
3330:.
3301:.
3271:.
3242:.
3213:)
3193:.
3179::
3156:.
3121:.
3092:.
3067:.
3047:.
3006:.
2972:.
2946:.
2944:)
2926:.
2913:.
2887:.
2885:)
2867:.
2854:.
2828:.
2826:)
2808:.
2795:.
2684:4
2678:O
2676:2
2674:C
2642:3
2617:4
2614:−
2595:3
2573:4
2551:3
2545:O
2543:2
2541:S
2525:2
2522:−
2519:O
2503:3
2481:4
2459:4
2437:2
2431:O
2415:4
2412:−
2393:4
2390:−
2371:2
2368:−
2349:3
2346:−
2327:4
2276:3
2273:−
2253:4
2250:−
2230:3
2227:−
2208:4
2205:−
2200:2
2198:H
2182:7
2176:O
2174:2
2156:4
2134:3
2131:−
2112:3
2094:)
2079:3
2076:−
2073:I
2057:2
2051:O
2035:3
2032:−
2029:N
1991:S
1977:O
1963:P
1949:N
1935:I
1921:H
1907:F
1879:C
1818:2
1800:O
1798:3
1796:H
1780:4
1777:+
1662:K
1517:H
1246:n
1241:n
1237:n
1207:H
1190:3
1187:+
1171:4
1168:+
1153:H
1148:3
1138:H
1132:.
1124:3
1121:−
1116:(
1067:+
1062:+
1001:e
997:+
948:e
944:+
939:+
731:.
468:/
462:.
456:ˌ
453:t
450:æ
447:k
444:ˈ
441:/
412:/
406:.
400:ˌ
397:n
394:æ
391:ˈ
388:/
341:.
150:2
120:K
87:/
78:n
75:ɒ
72:.
66:ˈ
63:/
59:(
34:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.