623:
615:
749:
264:, by treating calcium iodide or barium chloride with potassium or sodium bis(trimethylsilyl)amide. However, this method can result in potassium contamination. An improved synthesis involving the reaction of benzylpotassium with calcium iodide, followed by reaction with bis(trimethylsilyl)amine results in potassium-free material:
780:, which are monomeric in the gas phase and dimeric in the crystalline phase. Group 11 complexes are especially prone to oligomerization, forming tetramers in the solid phase. The Lewis acid properties of the group 12 complexes have been reported and the improved E and C numbers for the Zn and Cd complexes are listed in the
768:. In the solid state it forms a dimer with trigonal planar iron centers and bridging amido groups. The low coordination number of the iron complex is largely due to the steric effects of the bulky bis(trimethylsilyl)amide, however the complex will bind THF to give the adduct, {(THF)Fe
17:
91:
bulky complexes are molecular, consisting of mono-, di-, and tetramers. Having a built-in base, these compounds conveniently react with even weakly protic reagents. The class of ligands and pioneering studies on their coordination compounds were described by Bürger and
Wannagat.
166:
1907:
Driess, Matthias; Pritzkow, Hans; Skipinski, Markus; Winkler, Uwe (1997). "Synthesis and Solid State
Structures of Sterically Congested Sodium and Cesium Silyl(fluorosilyl)phosphanide Aggregates and Structural Characterization of the Trimeric Sodium Bis(trimethylsilyl)amide".
2872:
Wagner, Clifton L.; Phan, Nathan A.; Fettinger, James C.; Berben, Louise A.; Power, Philip P. (2019-04-05). "New
Characterization of V{N(SiMe 3 ) 2 } 3 : Reductions of Trisvanadium(III) and -chromium(III) To Afford the Reduced Metal(II) Anions − (M = V and Cr)".
3142:
Herrmann, Wolfgang A.; Anwander, Reiner; Kleine, Matthias; Scherer, Wolfgang (1992). "Lanthanoiden-Komplexe, I Solvensfreie
Alkoxid-Komplexe des Neodyms und Dysprosiums. Kristall- und Molekülstruktur von trans-Bis(acetonitril)tris(tri-tert-butylmethoxy)neodym".
630:
In line with the general method, bis(trimethylsilyl)amides of transition metals are prepared by a reaction between the metal halides (typically chlorides) and an alkali metal bis(trimethylsilyl)amide. There is some variation however, for instance the synthesis
1369:
However it is more common to see the preparation of lanthanide bis(trimethylsilyl)amides from anhydrous lanthanide chlorides, as these are cheaper. The reaction is performed in THF and requires a period at reflux. Once formed, the product is separated from
2500:
Andersen, R. A.; Faegri, Knut; Green, Jennifer C.; Haaland, Arne; Lappert, M. F.; Leung, Wing Por; Rypdal, Kristin (1 May 1988). "Synthesis of bisiron(II). Structure and bonding in M2 (M = manganese, iron, cobalt): two-coordinate transition-metal amides".
2256:
Bürger, H; Cichon, J; Goetze, U; Wannagat, U; Wismar, H.J (1971). "Beiträgezur chemie der silicium-stickstoff-verbindungen CVII. Darstellung, schwingungsspektren und normalkoordinatenanalyse von disilylamiden der 3. Gruppe: M3 mit M = Al, Ga und In".
2561:
Sulway, Scott A.; Collison, David; McDouall, Joseph J. W.; Tuna, Floriana; Layfield, Richard A. (21 March 2011). "Iron(II) Cage
Complexes of N-Heterocyclic Amide and Bis(trimethylsilyl)amide Ligands: Synthesis, Structure, and Magnetic Properties".
196:
Lithium, sodium, and potassium bis(trimethylsilyl)amides are commercially available. When free of solvent, the lithium and sodium complexes are trimeric, and the potassium complex is dimeric in solid state. The lithium reagent may be prepared from
1394:
Silylamides are important as starting materials in lanthanide chemistry, as lanthanide chlorides have either poor solubility or poor stability in common solvents. As a result of this nearly all lanthanide silylamides are commercially available.
2632:
Murray, Brendan D.; Power, Philip P. (1984). "Three-Coordinate Metal Amides of
Manganese(II) and Cobalt(II): Synthesis and X-ray Structure of the First Tris(silylamide) of Manganese and the X-ray Crystal Structures of (M = Mn, Co)".
2216:
Schaeffer, Charles D.; Myers, Lori K.; Coley, Suzanne M.; Otter, Julie C.; Yoder, Claude H. (1990). "Preparation, analysis, and reactivity of bistin(II): An advanced undergraduate laboratory project in organometallic synthesis".
2065:
Tanner, P. S.; Burkey, D. J.; Hanusa, T. P. (1995). "Cyclopentadienyl ring metathesis with bis(pentamethylcyclopentadienyl)calcium as a route to mixed ring organolanthanide complexes; the crystal structure of
3078:
Schuetz, Steven A.; Day, Victor W.; Sommer, Roger D.; Rheingold, Arnold L.; Belot, John A. (2001). "Anhydrous
Lanthanide Schiff Base Complexes and Their Preparation Using Lanthanide Triflate Derived Amides".
2995:
Ohki, Yasuhiro; Shimizu, Yuki; Araake, Ryoichi; Tada, Mizuki; Sameera, W. M. C.; Ito, Jun-Ichi; Nishiyama, Hisao (2016-12-19). "Co 6 H 8 (P i Pr 3 ) 6 : A Cobalt
Octahedron with Face-Capping Hydrides".
3198:
Avens, Larry R.; Bott, Simon G.; Clark, David L.; Sattelberger, Alfred P.; Watkin, John G.; Zwick, Bill D. (1993). "A Convenient Entry into
Trivalent Actinide Chemistry: Synthesis and Characterization of
2376:
Bradley, Donald C.; Copperthwaite, Richard G.; Extine, M. W.; Reichert, W. W.; Chisholm, Malcolm H. (1978). "Transition Metal
Complexes of Bis(Trimethyl-silyl)Amine (1,1,1,3,3,3-Hexamethyldisilazane)".
1687:
Metal bis(trimethylsilyl)amides are strong bases. They are corrosive, and are incompatible with many chlorinated solvents. These compounds react vigorously with water, and should be manipulated with
162:
formed as a by-product typically precipitates as a solid, allowing for its removal by filtration. The remaining metal bis(trimethylsilyl)amide is then often purified by distillation or sublimation.
127:
Apart from group 1 and 2 complexes, a general method for preparing metal bis(trimethylsilyl)amides entails reactions of anhydrous metal chloride with an alkali metal bis(trimethylsilyl)amides via a
315:; which is commercially available as a mixture of n-Bu and s-Bu isomers. It deprotonates the free amine to yield the magnesium bis(trimethylsilyl)amide, itself commercially available.
1592:
There has also been some success in the synthesis and characterization of actinide bis(trimethylsilyl)amides. A convenient synthetic route uses the THF-adducts of the iodide salts AnI
2927:
Ellison, Jeffrey J.; Power, Philip P.; Shoner, Steven C. (1989). "First Examples of Three-Coordinate Manganese(III) and Cobalt(III): Synthesis and Characterization of the Complexes M
3031:
Faust, Michelle; Bryan, Aimee M.; Mansikkamäki, Akseli; Vasko, Petra; Olmstead, Marilyn M.; Tuononen, Heikki M.; Grandjean, Fernande; Long, Gary J.; Power, Philip P. (2015-10-26).
685:, respectively. The melting and boiling points of the complexes decrease across the series, with Group 12 metals being sufficiently volatile to allow purification by distillation.
2113:
Johns, Adam M.; Chmely, Stephen C.; Hanusa, Timothy P. (2009). "Solution Interaction of Potassium and Calcium Bis(trimethylsilyl)amides; Preparation of Ca2from Dibenzylcalcium".
1965:
Amonoo-Neizer, E. H.; Shaw, R. A.; Skovlin, D. O.; Smith, B. C.; Rosenthal, Joel W.; Jolly, William L. (1966). "Lithium Bis(trimethylsilyl)amide and Tris(trimethylsilyl)amine".
2188:
Westerhausen, Matthias. (1991). "Synthesis and spectroscopic properties of bis(trimethylsilyl)amides of the alkaline-earth metals magnesium, calcium, strontium, and barium".
83:
Due to the bulky hydrocarbon backbone metal bis(trimethylsilyl)amide complexes have low lattice energies and are lipophilic. For this reason, they are soluble in a range of
393:
Tin(II) bis(trimethylsilyl)amide is prepared from anhydrous tin(II) chloride and is commercially available. It is used to prepare other metal bis(trimethylsilylamide)s via
2597:
Bradley, Donald C.; Hursthouse, Michael B.; Malik, K. M. Abdul; Möseler, Reinhold (1978). "The crystal molecular structure of "bis(hexamethyldisilylamido) manganese"".
343:
is not acidic enough to react with the group 2 metals, however complexes may be prepared via a reaction of tin(II) bis(trimethylsilyl)amide with the appropriate metal:
2285:
Vehkamäki, Marko; Hatanpää, Timo; Ritala, Mikko; Leskelä, Markku (2004). "Bismuth precursors for atomic layer deposition of bismuth-containing oxide films".
3117:"Low co-ordination numbers in lanthanide and actinide compounds. Part I. The preparation and characterization of tris{bis(trimethylsilyl)-amido}lanthanides"
2660:
James, Alicia M.; Laxman, Ravi K.; Fronczek, Frank R.; Maverick, Andrew W. (1998). "Phosphorescence and Structure of a Tetrameric Copper(I)-Amide Cluster".
3714:
248:
Alkali metal silylamides are soluble in a range of organic solvents, where they exist as aggregates, and are commonly used in organic chemistry as strong
3751:
2417:
Bürger, H; Sawodny, Wolfgang; Wannagat, Ulrich (1965). "Darstellung und schwinkungsspektren von silylamiden der elemente zink, cadmium und quecksilber".
1871:
Mootz, D.; Zinnius, A.; Böttcher, B. (1969). "Assoziation im festen Zustand von Bis(trimethylsilyl)amidolithium und Methyltrimethylsilanolatoberyllium".
2024:
3927:
3601:
3255:
1747:
400:
and bismuth(III) bis(trimethylsilyl)amides are prepared in the same manner; the aluminium complex may also be prepared by treating strongly basic
3945:
3781:
3950:
3818:
2835:
D. C. Bradley & R.G. Copperthwaite (1978). "Transition Metal Complexes of Bis(Trimethyl-silyl)Amine (1,1,1,3,3,3-Hexamethyldisilazane)".
760:
complex exists in two forms depending on its physical state. In the gas phase, the compound is a monomeric with two-coordinate Fe possessing
3837:
3908:
3632:
1937:
Tesh, Kris F.; Hanusa, Timothy P.; Huffman, John C. (1990). "Ion pairing in potassium: The x-ray crystal structure of unsolvated 2".
2779:
3568:
3773:
2312:
Maaninen, A.; Shvari, J.; Laitinen, R. S.; Chivers, T (2002). "Compounds of General Interest". In Coucouvanis, Dimitri (ed.).
3581:
3452:
2979:
2856:
2545:
2479:
2454:
1984:
1855:
1731:
1350:
2349:
Siivari, Jari; Chivers, Tristram; Laitinen, Risto S. (1993). "A simple, efficient synthesis of tetraselenium tetranitride".
561:, is a compound analogous to tetrasulfur tetranitride and can be synthesized by the reaction of selenium tetrachloride with
385:, adducts are formed. Hence non-coordinating solvents such as benzene or toluene must be used to obtain the free complexes.
3887:
3738:
3659:
3606:
3248:
3624:
3576:
381:
Long reaction times are required for this synthesis and when performed in the presence of coordinating solvents, such as
3971:
3899:
3854:
3810:
3794:
3596:
3563:
3553:
3440:
252:. They are also extensively used as precursors for the synthesis other bis(trimethylsilyl)amide complexes (see below).
191:
221:
The direct reaction of these molten metals with bis(trimethylsilyl)amine at high temperature has also been described:
3940:
3849:
3706:
3505:
2394:
2333:
3802:
3765:
3760:
3726:
3517:
3482:
3465:
3457:
3393:
3333:
3171:
Andersen, Richard A. (1979). "Tris((hexamethyldisilyl)amido)uranium(III): Preparation and Coordination Chemistry".
2153:; BW Skelton; AH White (1986). "Highly Hindered Amido-Lithium and Amido-Magnesium Complexes. Crystal-Structures of
183:
688:
Iron complexes are notable for having been isolated in both the ferrous (II) and ferric (III) oxidation states. Fe
3976:
3384:
3372:
3343:
3338:
3241:
2038:
Boncella, J. M.; Coston, C. J.; Cammack, J. K. (1991). "The synthesis of bis(hexamethyldisilylamido)barium(II)".
187:
3687:
3591:
3545:
3428:
3348:
3302:
3360:
3879:
3701:
3415:
84:
550:
2700:
2020:
3116:
1966:
401:
249:
3033:"The Instability of Ni{N(SiMe 3 ) 2 } 2 : A Fifty Year Old Transition Metal Silylamide Mystery"
2006:
573:. The latter compound is a metal bis(trimethylsilyl)amide and can be synthesized by the reaction of
58:
3863:
3423:
3278:
432:
340:
202:
128:
116:
1706:
3651:
504:
2596:
1802:
H. Bürger & U. Wannagat (1963). "Silylamido-Derivate von Chrom, Mangan, Nickel und Kupfer".
3746:
3558:
574:
3586:
1834:
Alfred R. Pray; Richard F. Heitmiller; Stanley Strycker (1990). "Anhydrous Metal Chlorides".
582:
3537:
3264:
2801:
Fisher, K. J.; Drago, R. S. (1975). "Trends in the Acidities of the Zinc Family Elements".
2227:
1881:
32:
692:
can be prepared by treating iron trichloride with lithium bis(trimethylsilyl)amide and is
8:
3197:
2659:
2560:
2419:
2317:
2259:
2231:
1885:
622:
3932:
3678:
3283:
3060:
2906:
2701:"Synthesis and X-ray molecular structures of the silver(I) amides [{Ag[μ-N(SiMe
2614:
1994:
1741:
1688:
724:
complex is prepared by treating iron dichloride with lithium bis(trimethylsilyl)amide:
87:, in contrast to simple metal halides, which only dissolve in reactive solvents. These
2431:
2271:
2051:
3097:
3052:
3013:
2975:
2898:
2890:
2852:
2783:
2677:
2579:
2541:
2475:
2450:
2390:
2329:
2131:
2099:
1980:
1851:
1727:
761:
444:
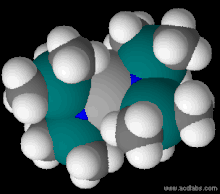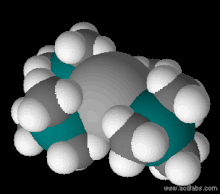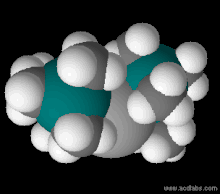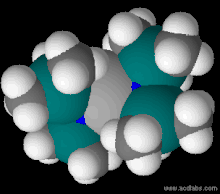
173:. Color scheme: H is white, Fe is gray, N is blue (barely visible), Si is blue-green.
2958:
Y. Ohki; S. Ohta & K. Tatsumi (2010). "Bio-Inspired Iron and Nickel Complexes".
2910:
2618:
3220:
3180:
3153:
3124:
3089:
3044:
3005:
2967:
2940:
2882:
2844:
2810:
2775:
2741:
2669:
2642:
2606:
2571:
2533:
2510:
2499:
2427:
2382:
2358:
2321:
2294:
2267:
2235:
2198:
2170:
2123:
2095:
2047:
1972:
1947:
1919:
1889:
1843:
1833:
1829:
1811:
1773:
1719:
394:
312:
3064:
2886:
1910:
1707:
382:
2697:
1764:
H. Bürger & U. Wannagat (1963). "Silylamido-Derivate von Eisen und Kobalt".
614:
3696:
3410:
2848:
2386:
2219:
2150:
1847:
675:
660:
198:
2971:
1976:
3965:
3405:
3310:
3157:
2963:
2894:
2840:
1893:
1839:
159:
3032:
3101:
3081:
3056:
3048:
3017:
3009:
2902:
2787:
2681:
2583:
2325:
2190:
2135:
2115:
1939:
1873:
1711:
693:
77:
2780:
10.1002/1521-3773(20000901)39:17<3082::AID-ANIE3082>3.0.CO;2-2
2537:
2037:
1723:
1353:
can be convenient anhydrous precursors to many bis(trimethylsilyl)amides:
3470:
3233:
3128:
2960:
Monomeric Iron(II) Complexes Having Two Sterically Hindered Arylthiolates
2745:
3224:
3184:
2944:
2926:
2814:
2646:
2514:
2362:
2320:. Vol. 33. New York: John Wiley & Sons, Inc. pp. 196–199.
2202:
1951:
3145:
3114:
2698:
Hitchcock, Peter B.; Lappert, Michael F.; Pierssens, Luc J.-M. (1996).
2610:
2064:
1815:
1777:
3093:
2673:
2575:
2239:
2174:
2127:
1923:
443:
S is prepared by the reaction of lithium bis(trimethylsilyl)amide and
2298:
2148:
1390:+ 3 LiCl + 3 butane (Ln = La, Ce, Pr, Nd, Sm, Eu, Gd, Ho, Yb, and Lu)
781:
618:
Frozen zinc bis(trimethylsilyl)amide. This compound melts at 12.5 °C.
2761:
626:
Titanium (left) and vanadium (right) tris{bis(trimethylsilyl)amide}.
2375:
20:
The bis(trimethylsilyl)amide ligand attached to a metal center M.
3115:
Bradley, Donald C.; Ghotra, Joginder S.; Hart, F. Alan (1973).
2834:
88:
36:
2284:
1906:
3141:
2762:
Bunge, Scott D.; Just, Oliver; Rees, William S. Jr (2000). "}
2311:
1964:
165:
40:
3030:
1371:
16:
2255:
748:
696:
as the high spin iron(III) contains 5 unpaired electrons.
3077:
2871:
2800:
260:
The calcium and barium complexes may be prepared via the
2957:
2215:
1374:
by exchanging the solvent for toluene, in which Ln(hmds)
76:
monovalent group, and are part of a broader category of
2837:
Transition Metal Complexes of Bis(trimethylsilyl)amine
2631:
2348:
2994:
2416:
3121:
Journal of the Chemical Society, Dalton Transactions
1870:
1801:
1763:
435:
entails the use of a metal bis(trimethylsilyl)amide
2699:
1828:Many metal chlorides may be dried by refluxing in
439:S as a precursor with pre-formed S–N bonds.
2112:
1936:
122:
3963:
339:In contrast to group 1 metals, the amine N-H in
655:are prepared using the soluble precursors TiCl
3249:
1365:+ 3 MOTf (M = Li, Na, K; Ln = La, Nd, Sm, Er)
2251:
2249:
2187:
1746:: CS1 maint: multiple names: authors list (
1238:Insoluble in hydrocarbons and diethyl ether
2922:
2920:
2495:
2493:
2491:
772:}. Similar behavior can be seen in Mn(hmds)
311:Magnesium silylamides can be prepared from
3263:
3256:
3242:
2830:
2828:
2826:
2824:
1797:
1795:
1793:
1791:
1789:
1787:
2693:
2691:
2527:
2469:
2246:
3170:
2917:
2488:
2474:. Hoboken, N.J.: John Wiley & Sons.
747:
621:
613:
606:) and lithium bis(trimethylsilyl)amide.
164:
15:
3037:Angewandte Chemie International Edition
2998:Angewandte Chemie International Edition
2821:
2768:Angewandte Chemie International Edition
2757:
2755:
2444:
2412:
2410:
2408:
2406:
1784:
3964:
2688:
2653:
1759:
1757:
43:bis(trimethylsilyl)amide ligands (the
3237:
2951:
2766:]: The First Base-Free Gold Amide".
2752:
2403:
1345:
609:
388:
255:
177:
3191:
3164:
3108:
1754:
752:Iron tris{bis(trimethylsilyl)amide}
471:The metal bis(trimethylsilyl)amide
261:
13:
2449:. Hoboken: John Wiley & Sons.
2149:LM Engelhardt; BS Jolly; PC Junk;
483:reacts with the combination of SCl
192:potassium bis(trimethylsilyl)amide
14:
3988:
2528:Rauchfuss, Thomas B, ed. (2010).
2843:Vol. 18. pp. 112–120.
1842:Vol. 28. pp. 321–323.
720:Similarly, the two coordinate Fe
184:lithium bis(trimethylsilyl)amide
3135:
3071:
3024:
2988:
2865:
2794:
2625:
2590:
2554:
2521:
2463:
2438:
2369:
2342:
2305:
2278:
2209:
2181:
2142:
2106:
2058:
2031:
1971:. Vol. 8. pp. 19–22.
188:sodium bis(trimethylsilyl)amide
25:Metal bis(trimethylsilyl)amides
2287:Journal of Materials Chemistry
2013:
1958:
1930:
1900:
1864:
1822:
1700:
1632:Sublimates at 80–100 °C (
1171:Tetrameric in the solid state
1103:Tetrameric in the solid state
123:General methods of preparation
119:from which they are prepared.
95:The ligands are often denoted
1:
2887:10.1021/acs.inorgchem.9b00381
2713:] and [{Ag[μ-NCMe
2470:Rauchfuss, Thomas B. (2010).
2432:10.1016/s0022-328x(00)84740-3
2381:. Vol. 18. p. 112.
2272:10.1016/s0022-328x(00)80797-4
2052:10.1016/s0277-5387(00)83767-5
1694:
2100:10.1016/0277-5387(94)00316-7
1386:+ 3 HMDS + 3 BuLi → Ln(hmds)
1378:is soluble but LiCl is not.
431:An alternative synthesis of
7:
2966:Vol. 35. p. 137.
1714:, Alexandra Seeber (2009).
851:105 °C/10 mmHg (subl.)
10:
3993:
2849:10.1002/9780470132494.ch18
2599:Transition Metal Chemistry
2445:Douglas, Bodie E. (1978).
2387:10.1002/9780470132494.ch18
1848:10.1002/9780470132593.ch80
1672:Sublimates at 60 °C (
1652:Sublimates at 60 °C (
551:Tetraselenium tetranitride
181:
3972:Bis(trimethylsilyl)amides
3920:
3830:
3671:
3644:
3530:
3498:
3326:
3295:
3271:
2972:10.1002/9780470651568.ch7
1977:10.1002/9780470132395.ch6
1682:
1262:
1195:
1152:
1085:
1036:
983:
953:
908:
864:
809:
487:and sulfuryl chloride (SO
402:lithium aluminium hydride
250:sterically hindered bases
169:Space-filling model of Fe
85:nonpolar organic solvents
3158:10.1002/cber.19921250902
1894:10.1002/ange.19690811015
433:tetrasulfur tetranitride
341:bis(trimethylsilyl)amine
203:bis(trimethylsilyl)amine
129:salt metathesis reaction
2738:Chemical Communications
2447:Inorganic Syntheses, 18
1718:. Weinheim: Wiley-VCH.
1600:as starting materials.
1289:Commercially available
860:Commercially available
505:trimethylsilyl chloride
404:with the parent amine:
3977:Coordination complexes
3265:Coordination complexes
3049:10.1002/anie.201505518
3010:10.1002/anie.201608262
2326:10.1002/0471224502.ch4
1804:Monatshefte für Chemie
1766:Monatshefte für Chemie
1361:+ 3 M(hmds) → Ln(hmds)
1209:180 / 0.2 mmHg (subl.)
1135:Dark olive green solid
1073:120 / 0.5 mmHg (subl.)
971:110 / 0.5 mmHg (subl.)
753:
627:
619:
575:selenium tetrachloride
507:, and sulfur dioxide:
174:
115:) in reference to the
33:coordination complexes
27:(often abbreviated as
21:
2538:10.1002/9780470651568
1724:10.1002/9780470740385
1716:Metal Amide Chemistry
1710:, Andrey Protchenko,
751:
625:
617:
583:selenium monochloride
420:→ Li(hmds) + Al(hmds)
168:
160:Alkali metal chloride
19:
3129:10.1039/DT9730001021
2746:10.1039/CC9960001189
1351:Lanthanide triflates
366:(M = Mg, Ca, Sr, Ba)
117:hexamethyldisilazane
3225:10.1021/ic00088a030
3213:Inorganic Chemistry
3211:(An = U, Np, Pu)".
3185:10.1021/ic50196a021
3173:Inorganic Chemistry
3043:(44): 12914–12917.
3004:(51): 15821–15825.
2945:10.1021/ja00202a071
2933:Inorganic Chemistry
2875:Inorganic Chemistry
2815:10.1021/ic50153a041
2803:Inorganic Chemistry
2662:Inorganic Chemistry
2647:10.1021/ic00194a036
2635:Inorganic Chemistry
2564:Inorganic Chemistry
2530:Inorganic Syntheses
2515:10.1021/ic00283a022
2503:Inorganic Chemistry
2472:Inorganic syntheses
2420:J. Organomet. Chem.
2379:Inorganic Syntheses
2363:10.1021/ic00060a031
2351:Inorganic Chemistry
2318:Inorganic Syntheses
2314:Inorganic Syntheses
2260:J. Organomet. Chem.
2232:1990JChEd..67..347S
2203:10.1021/ic00001a018
1968:Inorganic Syntheses
1952:10.1021/ic00333a029
1886:1969AngCh..81..398M
1836:Inorganic Syntheses
1263:Group 12 complexes
1196:Group 11 complexes
1153:Group 10 complexes
283:Ca(thf) + 2 HN(SiMe
217:→ Li(hmds) + butane
2611:10.1007/BF01393560
1816:10.1007/BF00904702
1810:(4–5): 1099–1102.
1778:10.1007/BF00905688
1689:air-free technique
1086:Group 9 complexes
1053:90-100 / 0.01 mmHg
1037:Group 8 complexes
984:Group 7 complexes
954:Group 6 complexes
909:Group 5 complexes
889:Prepared from TiCl
865:Group 4 complexes
810:Group 3 complexes
754:
628:
620:
175:
145:Na(hmds) → M(hmds)
22:
3959:
3958:
3219:(10): 2248–2256.
3123:(10): 1021–1023.
3094:10.1021/ic010060l
3088:(20): 5292–5295.
2981:978-0-470-65156-8
2939:(20): 8044–8046.
2931:(M = Mn or Co)".
2858:978-0-470-13249-4
2809:(11): 2804–2808.
2774:(17): 3082–3084.
2740:(10): 1189–1190.
2674:10.1021/ic971341p
2668:(15): 3785–3791.
2641:(26): 4584–4588.
2576:10.1021/ic102341a
2547:978-0-470-65156-8
2509:(10): 1782–1786.
2481:978-0-470-65156-8
2456:978-0-470-13284-5
2240:10.1021/ed067p347
2175:10.1071/CH9861337
2128:10.1021/ic8012766
1986:978-0-470-13239-5
1924:10.1021/om970444c
1918:(23): 5108–5112.
1857:978-0-470-13259-3
1733:978-0-470-72184-1
1680:
1679:
1590:
1589:
1346:f-Block complexes
1343:
1342:
1048:Light green solid
965:Apple-green solid
934:Prepared from VCl
920:Dark violet solid
876:Bright blue solid
756:The dark green Fe
610:d-Block complexes
445:sulfur dichloride
389:p-Block complexes
256:Group 2 complexes
178:Group 1 complexes
29:metal silylamides
3984:
3258:
3251:
3244:
3235:
3234:
3229:
3228:
3195:
3189:
3188:
3179:(6): 1507–1509.
3168:
3162:
3161:
3152:(9): 1971–1979.
3139:
3133:
3132:
3112:
3106:
3105:
3075:
3069:
3068:
3028:
3022:
3021:
2992:
2986:
2985:
2955:
2949:
2948:
2924:
2915:
2914:
2881:(9): 6095–6101.
2869:
2863:
2862:
2832:
2819:
2818:
2798:
2792:
2791:
2759:
2750:
2749:
2735:
2695:
2686:
2685:
2657:
2651:
2650:
2629:
2623:
2622:
2594:
2588:
2587:
2570:(6): 2521–2526.
2558:
2552:
2551:
2532:. Vol. 35.
2525:
2519:
2518:
2497:
2486:
2485:
2467:
2461:
2460:
2442:
2436:
2435:
2414:
2401:
2400:
2373:
2367:
2366:
2357:(8): 1519–1520.
2346:
2340:
2339:
2309:
2303:
2302:
2299:10.1039/b405891g
2282:
2276:
2275:
2253:
2244:
2243:
2213:
2207:
2206:
2185:
2179:
2178:
2146:
2140:
2139:
2122:(4): 1380–1384.
2110:
2104:
2103:
2062:
2056:
2055:
2035:
2029:
2028:
2027:
2023:
2017:
2011:
2010:
2004:
2000:
1998:
1990:
1962:
1956:
1955:
1946:(8): 1584–1586.
1934:
1928:
1927:
1904:
1898:
1897:
1868:
1862:
1861:
1830:thionyl chloride
1826:
1820:
1819:
1799:
1782:
1781:
1772:(6): 1007–1012.
1761:
1752:
1751:
1745:
1737:
1704:
1603:
1602:
1398:
1397:
1325:Colorless liquid
1300:Colorless liquid
1274:Colorless liquid
1068:Dark green solid
787:
786:
605:
604:
603:
595:
594:
572:
570:
569:
482:
480:
479:
395:transmetallation
357:
313:dibutylmagnesium
154:
149:
144:
139:
75:
59:monovalent anion
57:
3992:
3991:
3987:
3986:
3985:
3983:
3982:
3981:
3962:
3961:
3960:
3955:
3936:
3916:
3903:
3895:
3891:
3883:
3875:
3871:
3867:
3858:
3845:
3841:
3826:
3822:
3814:
3806:
3798:
3789:
3785:
3777:
3769:
3755:
3742:
3734:
3730:
3722:
3718:
3710:
3691:
3682:
3667:
3663:
3655:
3640:
3628:
3619:
3615:
3572:
3549:
3541:
3526:
3521:
3513:
3509:
3494:
3490:
3486:
3478:
3474:
3461:
3448:
3444:
3436:
3432:
3419:
3401:
3397:
3388:
3380:
3376:
3368:
3364:
3356:
3352:
3322:
3318:
3314:
3306:
3291:
3287:
3267:
3262:
3232:
3210:
3206:
3202:
3196:
3192:
3169:
3165:
3140:
3136:
3113:
3109:
3076:
3072:
3029:
3025:
2993:
2989:
2982:
2956:
2952:
2930:
2925:
2918:
2870:
2866:
2859:
2833:
2822:
2799:
2795:
2765:
2760:
2753:
2732:
2728:
2724:
2720:
2716:
2712:
2708:
2704:
2696:
2689:
2658:
2654:
2630:
2626:
2595:
2591:
2559:
2555:
2548:
2526:
2522:
2498:
2489:
2482:
2468:
2464:
2457:
2443:
2439:
2415:
2404:
2397:
2374:
2370:
2347:
2343:
2336:
2310:
2306:
2283:
2279:
2254:
2247:
2214:
2210:
2186:
2182:
2160:
2156:
2147:
2143:
2111:
2107:
2085:
2081:
2077:
2073:
2069:
2063:
2059:
2036:
2032:
2025:
2019:
2018:
2014:
2002:
2001:
1992:
1991:
1987:
1963:
1959:
1935:
1931:
1911:Organometallics
1905:
1901:
1880:(10): 398–399.
1869:
1865:
1858:
1827:
1823:
1800:
1785:
1762:
1755:
1739:
1738:
1734:
1708:Michael Lappert
1705:
1701:
1697:
1685:
1664:
1644:
1623:
1599:
1595:
1578:
1562:
1546:
1530:
1514:
1498:
1482:
1466:
1450:
1434:
1418:
1389:
1385:
1377:
1364:
1360:
1348:
1322:
1297:
1271:
1246:Colorless solid
1225:Colorless solid
1204:Colorless solid
1179:
1132:
1111:
1065:
1045:
1016:
992:
962:
949:
945:
941:
937:
917:
904:
900:
896:
892:
873:
842:
821:Colorless solid
818:
779:
775:
771:
765:
759:
743:
739:
735:
731:
723:
715:
711:
707:
703:
691:
684:
679:
673:
669:
664:
658:
654:
650:
646:
642:
638:
634:
612:
602:
599:
598:
597:
593:
590:
589:
588:
586:
580:
568:
565:
564:
563:
562:
560:
556:
546:
542:
538:
534:
530:
526:
522:
518:
514:
502:
498:
494:
490:
486:
478:
475:
474:
473:
472:
466:
462:
458:
450:
442:
438:
427:
423:
419:
415:
411:
391:
383:dimethoxyethane
376:
372:
365:
361:
355:
354:
350:
334:
330:
326:
322:
306:
302:
298:
294:
290:
286:
282:
275:
271:
258:
244:
240:
236:
232:
228:
216:
212:
194:
182:Main articles:
180:
172:
152:
150:
147:
142:
140:
137:
125:
114:
110:
106:
102:
74:
70:
66:
62:
56:
52:
48:
44:
12:
11:
5:
3990:
3980:
3979:
3974:
3957:
3956:
3954:
3953:
3948:
3943:
3938:
3934:
3930:
3924:
3922:
3921:Halide donors:
3918:
3917:
3915:
3914:
3906:
3901:
3897:
3893:
3889:
3885:
3881:
3877:
3873:
3869:
3865:
3861:
3856:
3852:
3847:
3843:
3839:
3834:
3832:
3828:
3827:
3825:
3824:
3820:
3816:
3812:
3808:
3804:
3800:
3796:
3792:
3787:
3783:
3779:
3775:
3771:
3767:
3763:
3758:
3753:
3749:
3744:
3740:
3736:
3732:
3728:
3724:
3720:
3716:
3712:
3708:
3704:
3699:
3694:
3689:
3685:
3680:
3675:
3673:
3669:
3668:
3666:
3665:
3661:
3657:
3653:
3648:
3646:
3642:
3641:
3639:
3638:
3630:
3626:
3622:
3617:
3613:
3609:
3604:
3599:
3594:
3589:
3584:
3579:
3574:
3570:
3566:
3561:
3556:
3551:
3547:
3543:
3539:
3534:
3532:
3528:
3527:
3525:
3524:
3519:
3515:
3511:
3507:
3502:
3500:
3496:
3495:
3493:
3492:
3488:
3484:
3480:
3476:
3472:
3468:
3463:
3459:
3455:
3450:
3446:
3442:
3438:
3434:
3430:
3426:
3421:
3417:
3413:
3408:
3403:
3399:
3395:
3391:
3386:
3382:
3378:
3374:
3370:
3366:
3362:
3358:
3354:
3350:
3346:
3341:
3336:
3330:
3328:
3324:
3323:
3321:
3320:
3316:
3312:
3308:
3304:
3299:
3297:
3293:
3292:
3290:
3289:
3285:
3281:
3275:
3273:
3269:
3268:
3261:
3260:
3253:
3246:
3238:
3231:
3230:
3208:
3204:
3200:
3190:
3163:
3134:
3107:
3070:
3023:
2987:
2980:
2950:
2928:
2916:
2864:
2857:
2820:
2793:
2763:
2751:
2730:
2726:
2722:
2718:
2714:
2710:
2706:
2702:
2687:
2652:
2624:
2605:(1): 253–254.
2589:
2553:
2546:
2520:
2487:
2480:
2462:
2455:
2437:
2426:(2): 113–120.
2402:
2395:
2368:
2341:
2334:
2304:
2277:
2245:
2220:J. Chem. Educ.
2208:
2180:
2158:
2154:
2141:
2105:
2094:(2): 331–333.
2083:
2079:
2075:
2071:
2067:
2057:
2046:(7): 769–770.
2030:
2012:
2003:|journal=
1985:
1957:
1929:
1899:
1863:
1856:
1821:
1783:
1753:
1732:
1698:
1696:
1693:
1684:
1681:
1678:
1677:
1670:
1668:
1665:
1662:
1658:
1657:
1650:
1648:
1645:
1642:
1638:
1637:
1630:
1627:
1624:
1621:
1617:
1616:
1613:
1610:
1607:
1597:
1593:
1588:
1587:
1585:
1582:
1579:
1576:
1572:
1571:
1569:
1566:
1563:
1560:
1556:
1555:
1553:
1550:
1547:
1544:
1540:
1539:
1537:
1534:
1531:
1528:
1524:
1523:
1521:
1518:
1515:
1512:
1508:
1507:
1505:
1502:
1499:
1496:
1492:
1491:
1489:
1486:
1483:
1480:
1476:
1475:
1473:
1470:
1467:
1464:
1460:
1459:
1457:
1454:
1451:
1448:
1444:
1443:
1441:
1438:
1435:
1432:
1428:
1427:
1425:
1422:
1419:
1416:
1412:
1411:
1408:
1405:
1402:
1392:
1391:
1387:
1383:
1375:
1367:
1366:
1362:
1358:
1347:
1344:
1341:
1340:
1338:
1332:
1331:78 / 0.15 mmHg
1329:
1326:
1323:
1320:
1316:
1315:
1313:
1307:
1304:
1301:
1298:
1295:
1291:
1290:
1287:
1281:
1278:
1275:
1272:
1269:
1265:
1264:
1260:
1259:
1257:
1251:
1249:
1247:
1244:
1240:
1239:
1236:
1230:
1228:
1226:
1223:
1219:
1218:
1216:
1210:
1207:
1205:
1202:
1198:
1197:
1193:
1192:
1190:
1188:
1185:
1183:
1180:
1177:
1173:
1172:
1169:
1167:
1165:
1162:
1159:
1155:
1154:
1150:
1149:
1147:
1141:
1139:
1136:
1133:
1130:
1126:
1125:
1123:
1121:
1120:101 / 0.6 mmHg
1118:
1115:
1112:
1109:
1105:
1104:
1101:
1099:
1097:
1095:
1092:
1088:
1087:
1083:
1082:
1080:
1074:
1071:
1069:
1066:
1063:
1059:
1058:
1056:
1054:
1051:
1049:
1046:
1043:
1039:
1038:
1034:
1033:
1031:
1025:
1023:
1020:
1017:
1014:
1010:
1009:
1007:
1001:
1000:100 / 0.2 mmHg
998:
996:
993:
990:
986:
985:
981:
980:
978:
972:
969:
966:
963:
960:
956:
955:
951:
950:
947:
943:
939:
935:
932:
926:
924:
921:
918:
915:
911:
910:
906:
905:
902:
898:
894:
890:
887:
881:
879:
877:
874:
871:
867:
866:
862:
861:
858:
852:
849:
846:
843:
840:
836:
835:
833:
827:
825:
822:
819:
816:
812:
811:
807:
806:
803:
800:
797:
794:
791:
777:
773:
769:
763:
757:
746:
745:
741:
737:
733:
729:
721:
718:
717:
713:
709:
705:
701:
689:
682:
677:
671:
667:
662:
656:
652:
648:
644:
640:
636:
632:
611:
608:
600:
591:
578:
566:
558:
554:
548:
547:
544:
540:
536:
532:
528:
524:
520:
516:
512:
500:
496:
492:
488:
484:
476:
469:
468:
464:
460:
456:
448:
440:
436:
429:
428:
425:
421:
417:
413:
409:
397:. The group 13
390:
387:
379:
378:
374:
370:
367:
363:
359:
352:
348:
337:
336:
332:
328:
324:
323:Mg + 2 HN(SiMe
320:
309:
308:
304:
300:
296:
292:
288:
284:
280:
277:
273:
269:
262:general method
257:
254:
246:
245:
242:
238:
234:
230:
226:
219:
218:
214:
210:
209:BuLi + HN(SiMe
199:n-butyllithium
179:
176:
170:
157:
156:
146:
136:
124:
121:
112:
108:
104:
100:
99:(e.g. M(N(SiMe
72:
68:
64:
54:
50:
46:
35:composed of a
9:
6:
4:
3:
2:
3989:
3978:
3975:
3973:
3970:
3969:
3967:
3952:
3949:
3947:
3944:
3942:
3939:
3937:
3931:
3929:
3926:
3925:
3923:
3919:
3913:
3912:
3907:
3905:
3898:
3896:
3886:
3884:
3878:
3876:
3862:
3860:
3853:
3851:
3848:
3846:
3836:
3835:
3833:
3829:
3823:
3817:
3815:
3809:
3807:
3801:
3799:
3793:
3791:
3780:
3778:
3772:
3770:
3764:
3762:
3759:
3757:
3750:
3748:
3745:
3743:
3737:
3735:
3725:
3723:
3713:
3711:
3705:
3703:
3700:
3698:
3695:
3693:
3686:
3684:
3677:
3676:
3674:
3670:
3664:
3658:
3656:
3650:
3649:
3647:
3643:
3637:
3635:
3631:
3629:
3623:
3621:
3610:
3608:
3605:
3603:
3600:
3598:
3595:
3593:
3590:
3588:
3585:
3583:
3580:
3578:
3575:
3573:
3567:
3565:
3562:
3560:
3557:
3555:
3552:
3550:
3544:
3542:
3536:
3535:
3533:
3529:
3523:
3516:
3514:
3504:
3503:
3501:
3497:
3491:
3481:
3479:
3469:
3467:
3464:
3462:
3456:
3454:
3451:
3449:
3439:
3437:
3427:
3425:
3422:
3420:
3414:
3412:
3409:
3407:
3404:
3402:
3392:
3390:
3383:
3381:
3371:
3369:
3359:
3357:
3347:
3345:
3342:
3340:
3337:
3335:
3332:
3331:
3329:
3325:
3319:
3309:
3307:
3301:
3300:
3298:
3294:
3288:
3282:
3280:
3277:
3276:
3274:
3270:
3266:
3259:
3254:
3252:
3247:
3245:
3240:
3239:
3236:
3226:
3222:
3218:
3214:
3194:
3186:
3182:
3178:
3174:
3167:
3159:
3155:
3151:
3148:
3147:
3138:
3130:
3126:
3122:
3118:
3111:
3103:
3099:
3095:
3091:
3087:
3084:
3083:
3074:
3066:
3062:
3058:
3054:
3050:
3046:
3042:
3038:
3034:
3027:
3019:
3015:
3011:
3007:
3003:
2999:
2991:
2983:
2977:
2973:
2969:
2965:
2964:Inorg. Synth.
2961:
2954:
2946:
2942:
2938:
2934:
2923:
2921:
2912:
2908:
2904:
2900:
2896:
2892:
2888:
2884:
2880:
2876:
2868:
2860:
2854:
2850:
2846:
2842:
2841:Inorg. Synth.
2838:
2831:
2829:
2827:
2825:
2816:
2812:
2808:
2804:
2797:
2789:
2785:
2781:
2777:
2773:
2769:
2758:
2756:
2747:
2743:
2739:
2734:
2694:
2692:
2683:
2679:
2675:
2671:
2667:
2663:
2656:
2648:
2644:
2640:
2636:
2628:
2620:
2616:
2612:
2608:
2604:
2600:
2593:
2585:
2581:
2577:
2573:
2569:
2565:
2557:
2549:
2543:
2539:
2535:
2531:
2524:
2516:
2512:
2508:
2504:
2496:
2494:
2492:
2483:
2477:
2473:
2466:
2458:
2452:
2448:
2441:
2433:
2429:
2425:
2422:
2421:
2413:
2411:
2409:
2407:
2398:
2396:9780470132494
2392:
2388:
2384:
2380:
2372:
2364:
2360:
2356:
2352:
2345:
2337:
2335:9780471208259
2331:
2327:
2323:
2319:
2315:
2308:
2300:
2296:
2292:
2288:
2281:
2273:
2269:
2265:
2262:
2261:
2252:
2250:
2241:
2237:
2233:
2229:
2225:
2222:
2221:
2212:
2204:
2200:
2196:
2193:
2192:
2184:
2176:
2172:
2168:
2164:
2163:Aust. J. Chem
2152:
2145:
2137:
2133:
2129:
2125:
2121:
2118:
2117:
2109:
2101:
2097:
2093:
2089:
2061:
2053:
2049:
2045:
2041:
2034:
2022:
2016:
2008:
1996:
1988:
1982:
1978:
1974:
1970:
1969:
1961:
1953:
1949:
1945:
1942:
1941:
1933:
1925:
1921:
1917:
1913:
1912:
1903:
1895:
1891:
1887:
1883:
1879:
1876:
1875:
1867:
1859:
1853:
1849:
1845:
1841:
1840:Inorg. Synth.
1837:
1831:
1825:
1817:
1813:
1809:
1805:
1798:
1796:
1794:
1792:
1790:
1788:
1779:
1775:
1771:
1767:
1760:
1758:
1749:
1743:
1735:
1729:
1725:
1721:
1717:
1713:
1709:
1703:
1699:
1692:
1690:
1675:
1671:
1669:
1667:Yellow-orange
1666:
1660:
1659:
1655:
1651:
1649:
1646:
1640:
1639:
1635:
1631:
1628:
1625:
1619:
1618:
1614:
1611:
1608:
1605:
1604:
1601:
1586:
1583:
1580:
1574:
1573:
1570:
1567:
1564:
1558:
1557:
1554:
1551:
1548:
1542:
1541:
1538:
1535:
1532:
1526:
1525:
1522:
1519:
1516:
1510:
1509:
1506:
1503:
1500:
1494:
1493:
1490:
1487:
1484:
1478:
1477:
1474:
1471:
1468:
1462:
1461:
1458:
1455:
1452:
1446:
1445:
1442:
1439:
1436:
1430:
1429:
1426:
1423:
1420:
1414:
1413:
1409:
1406:
1403:
1400:
1399:
1396:
1381:
1380:
1379:
1373:
1356:
1355:
1354:
1352:
1339:
1336:
1333:
1330:
1327:
1324:
1318:
1317:
1314:
1311:
1308:
1306:93 / 0.5 mmHg
1305:
1302:
1299:
1293:
1292:
1288:
1285:
1282:
1280:82 / 0.5 mmHg
1279:
1276:
1273:
1267:
1266:
1261:
1258:
1255:
1252:
1250:
1248:
1245:
1242:
1241:
1237:
1234:
1231:
1229:
1227:
1224:
1221:
1220:
1217:
1214:
1211:
1208:
1206:
1203:
1200:
1199:
1194:
1191:
1189:
1187:80 / 0.2 mmHg
1186:
1184:
1181:
1175:
1174:
1170:
1168:
1166:
1163:
1160:
1157:
1156:
1151:
1148:
1145:
1142:
1140:
1137:
1134:
1128:
1127:
1124:
1122:
1119:
1116:
1113:
1107:
1106:
1102:
1100:
1098:
1096:
1093:
1090:
1089:
1084:
1081:
1078:
1075:
1072:
1070:
1067:
1061:
1060:
1057:
1055:
1052:
1050:
1047:
1041:
1040:
1035:
1032:
1029:
1026:
1024:
1021:
1018:
1012:
1011:
1008:
1005:
1002:
999:
997:
994:
988:
987:
982:
979:
976:
973:
970:
967:
964:
958:
957:
952:
933:
930:
927:
925:
922:
919:
913:
912:
907:
888:
885:
882:
880:
878:
875:
869:
868:
863:
859:
856:
853:
850:
847:
844:
838:
837:
834:
831:
828:
826:
823:
820:
814:
813:
808:
804:
801:
798:
795:
792:
789:
788:
785:
783:
767:
750:
727:
726:
725:
699:
698:
697:
695:
686:
680:
665:
624:
616:
607:
584:
576:
552:
510:
509:
508:
506:
454:
453:
452:
446:
434:
407:
406:
405:
403:
399:
396:
386:
384:
368:
347:M + 2 HN(SiMe
346:
345:
344:
342:
318:
317:
316:
314:
278:
267:
266:
265:
263:
253:
251:
224:
223:
222:
208:
207:
206:
204:
200:
193:
189:
185:
167:
163:
161:
134:
133:
132:
130:
120:
118:
98:
93:
90:
86:
81:
79:
60:
42:
39:metal M with
38:
34:
30:
26:
18:
3910:
3633:
3611:
3216:
3212:
3193:
3176:
3172:
3166:
3149:
3144:
3137:
3120:
3110:
3085:
3082:Inorg. Chem.
3080:
3073:
3040:
3036:
3026:
3001:
2997:
2990:
2959:
2953:
2936:
2932:
2878:
2874:
2867:
2836:
2806:
2802:
2796:
2771:
2767:
2737:
2665:
2661:
2655:
2638:
2634:
2627:
2602:
2598:
2592:
2567:
2563:
2556:
2529:
2523:
2506:
2502:
2471:
2465:
2446:
2440:
2423:
2418:
2378:
2371:
2354:
2350:
2344:
2313:
2307:
2293:(21): 3191.
2290:
2286:
2280:
2263:
2258:
2223:
2218:
2211:
2194:
2191:Inorg. Chem.
2189:
2183:
2166:
2162:
2144:
2119:
2116:Inorg. Chem.
2114:
2108:
2091:
2087:
2060:
2043:
2039:
2033:
2015:
1967:
1960:
1943:
1940:Inorg. Chem.
1938:
1932:
1915:
1909:
1902:
1877:
1874:Angew. Chem.
1872:
1866:
1835:
1824:
1807:
1803:
1769:
1765:
1715:
1712:Philip Power
1702:
1686:
1673:
1653:
1633:
1591:
1437:Yellow-brown
1393:
1368:
1349:
1334:
1309:
1283:
1253:
1232:
1212:
1161:Black solid
1143:
1094:Black Solid
1076:
1027:
1019:Violet solid
1003:
974:
928:
883:
854:
829:
776:and Co(hmds)
755:
719:
694:paramagnetic
687:
643:and V{N(SiMe
629:
549:
470:
430:
398:
392:
380:
369:M + Sn(hmds)
338:
310:
276:Ca(thf) + KI
259:
247:
220:
195:
158:
126:
96:
94:
82:
78:metal amides
28:
24:
23:
2169:(9): 1337.
1485:Pale yellow
1114:Green solid
995:Beige solid
845:White solid
732:+ 2LiN(SiMe
704:+ 3LiN(SiMe
495:) to form S
412:+ 4 HN(SiMe
268:2 BnK + CaI
225:M + HN(SiMe
3966:Categories
3582:amino acid
3499:Si donors:
3146:Chem. Ber.
2226:(4): 347.
2197:: 96–101.
2088:Polyhedron
2040:Polyhedron
2021:US 5420322
1695:References
1647:Blue-black
1626:Red-purple
1609:Appearance
1533:Pale green
1453:Pale green
1404:Appearance
1182:Red liquid
793:Appearance
543:SiCl + 2SO
467:S + 2 LiCl
335:+ 2 butane
331:→ Mg(hmds)
291:→ Ca(hmds)
272:+ THF → Bn
3831:S donors:
3672:O donors:
3645:P donors:
3607:porphyrin
3554:imidazole
3531:N donors:
3327:C donors:
3296:B donors:
3272:H donors:
2895:0020-1669
2151:CL Raston
2005:ignored (
1995:cite book
1742:cite book
1676:10 torr)
1656:10 torr)
1636:10 torr)
1612:m.p. (°C)
1469:Pale blue
1407:m.p. (°C)
1158:Ni(hmds)
1091:Co(hmds)
799:b.p. (°C)
796:m.p. (°C)
782:ECW model
631:Ti{N(SiMe
459:NLi + SCl
373:→ M(hmds)
233:→ MN(SiMe
111:= M(hmds)
3102:11559096
3057:26332337
3018:27862749
2911:96436047
2903:30950608
2788:11028039
2682:11670480
2619:95499411
2584:21314147
2266:: 1–12.
2136:19138130
1661:Pu(hmds)
1641:Np(hmds)
1615:Comment
1606:Compound
1575:Lu(hmds)
1559:Yb(hmds)
1543:Ho(hmds)
1527:Dy(hmds)
1511:Gd(hmds)
1495:Eu(hmds)
1479:Sm(hmds)
1463:Nd(hmds)
1447:Pr(hmds)
1431:Ce(hmds)
1415:La(hmds)
1410:Comment
1401:Compound
1319:Hg(hmds)
1294:Cd(hmds)
1268:Zn(hmds)
1243:Au(hmds)
1222:Ag(hmds)
1201:Cu(hmds)
1176:Ni(hmds)
1164:>250
1129:Co(hmds)
1108:Co(hmds)
1062:Fe(hmds)
1042:Fe(hmds)
1013:Mn(hmds)
989:Mn(hmds)
959:Cr(hmds)
870:Ti(hmds)
815:Sc(hmds)
805:Comment
790:Compound
766:symmetry
515:S + 2SCl
63:−N(Si(CH
37:cationic
3445:& C
2228:Bibcode
1882:Bibcode
1629:137–140
1620:U(hmds)
1584:167-170
1568:162-165
1552:161-164
1536:157–160
1520:160-163
1504:159-162
1488:155-158
1472:161-164
1456:155-158
1440:132-140
1424:145-149
1357:Ln(OTf)
1022:108-110
923:174-176
914:V(hmds)
848:180-184
839:Y(hmds)
824:172-174
744:+ 2LiCl
716:+ 3LiCl
535:+ 8 (CH
358:M(hmds)
241:+ 1/2 H
45:N(Si(CH
41:anionic
3353:=CH-CH
3207:and An
3100:
3065:884563
3063:
3055:
3016:
2978:
2909:
2901:
2893:
2855:
2786:
2733:]"
2729:]}
2709:]}
2680:
2617:
2582:
2544:
2478:
2453:
2393:
2332:
2134:
2026:
1983:
1854:
1832:. See
1730:
1683:Safety
1565:Yellow
1501:Orange
1382:Ln(Cl)
670:or VCl
190:, and
89:steric
31:) are
3344:HC(O)
3339:RC(O)
3203:(THF)
3061:S2CID
2907:S2CID
2615:S2CID
1596:(THF)
1581:White
1549:Cream
1517:White
1421:White
1138:86-88
1079:= 5/2
1006:= 5/2
977:= 3/2
938:(N(CH
893:(N(CH
886:= 1/2
577:(SeCl
519:+ 2SO
424:+ 4 H
408:LiAlH
307:+ THF
295:+ 2 C
61:, or
3747:acac
3719:/HCO
3602:bipy
3361:C(CH
3098:PMID
3053:PMID
3014:PMID
2976:ISBN
2899:PMID
2891:ISSN
2853:ISBN
2784:PMID
2678:PMID
2580:PMID
2542:ISBN
2476:ISBN
2451:ISBN
2391:ISBN
2330:ISBN
2157:and
2132:PMID
2086:)".
2078:Nd(C
2007:help
1981:ISBN
1852:ISBN
1748:link
1728:ISBN
1372:LiCl
1277:12.5
802:Spin
740:→ Fe
728:FeCl
712:→ Fe
700:FeCl
553:, Se
447:(SCl
377:+ Sn
201:and
155:NaCl
97:hmds
3842:NCS
3819:OPR
3795:OSR
3774:ClO
3761:ONO
3739:RCO
3616:Si)
3612:(Me
3597:RCN
3564:RNO
3512:4−n
3510:SiR
3466:≡CR
3458:=CR
3453:RNC
3377:=CH
3221:doi
3199:AnI
3181:doi
3154:doi
3150:125
3125:doi
3090:doi
3045:doi
3006:doi
2968:doi
2941:doi
2937:111
2883:doi
2845:doi
2811:doi
2776:doi
2742:doi
2725:CMe
2717:(CH
2670:doi
2643:doi
2607:doi
2572:doi
2534:doi
2511:doi
2428:doi
2383:doi
2359:doi
2322:doi
2295:doi
2268:doi
2236:doi
2199:doi
2171:doi
2161:".
2124:doi
2096:doi
2048:doi
1973:doi
1948:doi
1920:doi
1890:doi
1844:doi
1812:doi
1774:doi
1720:doi
1674:ca.
1654:ca.
1634:ca.
1337:= 0
1312:= 0
1286:= 0
1256:= 0
1235:= 0
1215:= 0
1146:= 2
1030:= 2
968:120
931:= 1
857:= 0
832:= 0
676:NMe
661:NMe
581:),
527:→ S
451:).
362:+ H
135:MCl
3968::
3946:Br
3941:Cl
3909:NC
3900:SR
3880:SO
3850:RS
3811:PO
3803:SO
3790:NO
3766:NO
3756:CO
3715:CO
3697:RO
3660:PR
3652:PR
3636:CS
3592:RN
3577:py
3569:NO
3559:NO
3538:NH
3522:Si
3447:70
3443:60
3416:CO
3411:CO
3406:CN
3385:RC
3373:CH
3349:CH
3303:BR
3217:33
3215:.
3177:18
3175:.
3119:.
3096:.
3086:40
3059:.
3051:.
3041:54
3039:.
3035:.
3012:.
3002:55
3000:.
2974:.
2962:.
2935:.
2919:^
2905:.
2897:.
2889:.
2879:58
2877:.
2851:.
2839:.
2823:^
2807:14
2805:.
2782:.
2772:39
2770:.
2754:^
2736:.
2690:^
2676:.
2666:37
2664:.
2639:23
2637:.
2613:.
2601:.
2578:.
2568:50
2566:.
2540:.
2507:27
2505:.
2490:^
2405:^
2389:.
2355:32
2353:.
2328:.
2316:.
2291:14
2289:.
2264:33
2248:^
2234:.
2224:67
2195:30
2167:39
2165:.
2130:.
2120:48
2092:14
2090:.
2070:Me
2066:(C
2044:10
2042:.
1999::
1997:}}
1993:{{
1979:.
1944:29
1916:16
1914:.
1888:.
1878:81
1850:.
1838:.
1808:95
1806:.
1786:^
1770:94
1768:.
1756:^
1744:}}
1740:{{
1726:.
1691:.
1328:11
1117:73
784:.
596:Cl
587:Se
571:Se
523:Cl
503:,
491:Cl
463:→
455:2
319:Bu
303:CH
279:Bn
205::
186:,
151:+
141:+
131::
80:.
3951:I
3935:2
3933:F
3928:F
3911:S
3904:O
3902:2
3894:3
3892:O
3890:2
3888:S
3882:2
3874:2
3872:S
3870:2
3868:C
3866:2
3864:R
3859:S
3857:2
3855:R
3844:2
3840:2
3838:R
3821:3
3813:4
3805:4
3797:2
3788:5
3786:H
3784:5
3782:C
3776:4
3768:3
3754:2
3752:R
3741:2
3733:4
3731:O
3729:2
3727:C
3721:3
3717:3
3709:2
3707:O
3702:O
3692:O
3690:2
3688:R
3683:O
3681:2
3679:H
3662:2
3654:3
3634:N
3627:2
3625:N
3620:N
3618:2
3614:3
3587:N
3571:2
3548:3
3546:N
3540:3
3520:3
3518:R
3508:n
3506:H
3489:7
3487:H
3485:9
3483:C
3477:5
3475:H
3473:5
3471:C
3460:2
3441:C
3435:6
3433:R
3431:6
3429:C
3424:C
3418:2
3400:4
3398:H
3396:6
3394:C
3389:R
3387:2
3379:2
3375:2
3367:3
3365:)
3363:2
3355:2
3351:2
3334:R
3317:n
3315:H
3313:m
3311:B
3305:2
3286:2
3284:H
3279:H
3257:e
3250:t
3243:v
3227:.
3223::
3209:3
3205:4
3201:3
3187:.
3183::
3160:.
3156::
3131:.
3127::
3104:.
3092::
3067:.
3047::
3020:.
3008::
2984:.
2970::
2947:.
2943::
2929:3
2913:.
2885::
2861:.
2847::
2817:.
2813::
2790:.
2778::
2764:4
2748:.
2744::
2731:4
2727:2
2723:3
2721:)
2719:2
2715:2
2711:4
2707:2
2705:)
2703:3
2684:.
2672::
2649:.
2645::
2621:.
2609::
2603:3
2586:.
2574::
2550:.
2536::
2517:.
2513::
2484:.
2459:.
2434:.
2430::
2424:3
2399:.
2385::
2365:.
2361::
2338:.
2324::
2301:.
2297::
2274:.
2270::
2242:.
2238::
2230::
2205:.
2201::
2177:.
2173::
2159:2
2155:2
2138:.
2126::
2102:.
2098::
2084:5
2082:H
2080:5
2076:2
2074:)
2072:5
2068:5
2054:.
2050::
2009:)
1989:.
1975::
1954:.
1950::
1926:.
1922::
1896:.
1892::
1884::
1860:.
1846::
1818:.
1814::
1780:.
1776::
1750:)
1736:.
1722::
1663:3
1643:3
1622:3
1598:4
1594:3
1577:3
1561:3
1545:3
1529:3
1513:3
1497:3
1481:3
1465:3
1449:3
1433:3
1417:3
1388:3
1384:3
1376:3
1363:3
1359:3
1335:S
1321:2
1310:S
1303:8
1296:2
1284:S
1270:2
1254:S
1233:S
1213:S
1178:2
1144:S
1131:3
1110:2
1077:S
1064:3
1044:2
1028:S
1015:3
1004:S
991:2
975:S
961:3
948:2
946:)
944:3
942:)
940:3
936:3
929:S
916:3
903:2
901:)
899:3
897:)
895:3
891:3
884:S
872:3
855:S
841:3
830:S
817:3
778:2
774:2
770:2
764:4
762:S
758:2
742:2
738:2
736:)
734:3
730:2
722:2
714:3
710:2
708:)
706:3
702:3
690:3
683:2
681:)
678:3
674:(
672:3
668:2
666:)
663:3
659:(
657:3
653:3
651:}
649:2
647:)
645:3
641:3
639:}
637:2
635:)
633:3
601:2
592:2
585:(
579:4
567:2
559:4
557:N
555:4
545:2
541:3
539:)
537:3
533:4
531:N
529:4
525:2
521:2
517:2
513:2
511:2
501:4
499:N
497:4
493:2
489:2
485:2
481:S
477:2
465:2
461:2
457:2
449:2
441:2
437:2
426:2
422:3
418:2
416:)
414:3
410:4
375:2
371:2
364:2
360:2
356:↛
353:2
351:)
349:3
333:2
329:2
327:)
325:3
321:2
305:3
301:5
299:H
297:6
293:2
289:2
287:)
285:3
281:2
274:2
270:2
243:2
239:2
237:)
235:3
231:2
229:)
227:3
215:2
213:)
211:3
171:2
153:n
148:n
143:n
138:n
113:3
109:3
107:)
105:2
103:)
101:3
73:2
71:)
69:3
67:)
65:3
55:2
53:)
51:3
49:)
47:3
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.