207:
222:(the type of milk produced at the end stages of pregnancy), providing much needed immune support to newly born infants. It was widely believed that lactoferrin was only a bacteriostatic agent due to its high iron affinity and its ability to sequester free iron atoms from pathogenic microbes. It is now known, however, that the major antimicrobial driving force lies in the bactericidal properties of its iron-bound pocket and a specific peptide lactoferricin located at the N-lobe. Lactoferrin is able to bind to the LPS (
94:
four nitrogen atoms of a porphyrin ring. Along with a histidine, the apo form has five ligands surrounding the iron atom. Oxygen binds to the empty sixth position to form an octahedral complex in the holo form. Oxygen binding is fully cooperative for each of the subunits because as the first oxygen binds to one of the four heme groups, the protein undergoes a drastic conformational change that sharply increases the oxygen affinity of the other three subunits.
164:
86:
203:
concentrations. The tertiary structure is composed of two lobes, termed N and C lobes, each containing one iron-binding pocket. Each pocket contributes four amino acids (two tyrosines, one histidine, and one aspartate) and, along with two carbonate or bicarbonate anions, forms a six-membered coordinate around the iron cation. It is this specific combination that makes lactoferrin's iron affinity 300 times greater than transferrin.
235:
51:
226:) layer of bacteria, and in its holo form the iron atom oxidizes the lipopolysaccharides to lyse the outer membrane and simultaneously produce toxic hydrogen peroxide. Additionally, upon cleavage of lactoferrin by trypsin, the peptide lactoferricin is produced which binds to H-ATPase, disrupting proton translocation and ultimately killing the cell.
129:
shuttles by switching the oxidation state of the heme iron atom between ferrous (Fe) and ferric (Fe). Various cytochromes in combination with other redox-active molecules form a gradient of standard reduction potentials that increases the efficiency of energy coupling during electron-transfer events.
93:
Hemoglobin is an oxygen-transport protein found in virtually all vertebrates. Hemoglobin A is the main type found in human adults. It is a tetramer consisting of two alpha and two beta subunits. Each of the four monomeric units contain a heme prosthetic group in which a ferric cation is bound between
62:
ring coordinated with an iron ion. Four nitrogen atoms in the porphyrin ring act as a ligand for the iron in the center. In many cases, the equatorial porphyrin is complemented by one or two axial ligands. An example of this is in hemoglobin, where the porphyrin works together with a histidine side
101:
partial pressure. Fetal hemoglobin is a variant containing two gamma subunits instead of two beta subunits. Fetal hemoglobin is the predominant form up until the infant is several months old, and it has a greater oxygen affinity to compensate for the low oxygen tension of supplied maternal blood
248:
Ferritin is an iron reservoir for an individual cell. It is found in all cells types and localized in the cytosol. Ferritin is a large protein composed of 24 subunits surrounding a core full of iron atoms. It is capable of holding 0-4500 iron atoms, which can be used as a reservoir for cellular
128:
Cytochromes are heme-containing enzymes that act as single-electron transporters, most notably as electron shuttles in oxidative phosphorylation and photosynthesis. Types of well-studied cytochromes include cytochromes a-c, cytochrome oxidase, and cytochrome P450. These proteins act as electron
37:
Iron-dependent enzymes catalyze a variety of biochemical reactions and can be divided into three broad classes depending on the structure of their active site: non-heme mono-iron, non-heme diiron , or heme centers. A well-known family of iron-dependent enzymes include oxygenases that facilitate
202:
Lactoferrin is a member of the transferrin family and is the predominant protein found in mammal exocrine secretions, such as tears, milk, and saliva. It is composed of approximately 700 residues and exists mainly as a tetramer, with the monomer:tetramer ratio being 1:4 at 10 μM protein
185:. Transferrin can bind two Fe(III) ions, along with an anion (usually carbonate). To release the iron, the carbonate anion is protonated. This changes the carbonate's interaction with the protein, changing the conformation and allowing Fe(III) to be transferred.
249:
needs. Iron is stored when there is excess, and retrieved when iron is needed again. The subunits are a mixture of H (heavy or heart) and L (light or liver). The subunits form a cluster 70-80 Angstroms wide, which is then filled with iron ferrihydrite.
143:
Iron-sulfur proteins are those with an iron structure that includes sulfur. There are a variety of forms iron and sulfur can take in proteins, but the most common are and . Clusters are often associated with cysteine residues in the protein chain.
256:
Ferritin is used to diagnose low iron levels in humans. It can be used to indicate the level of bioavailable iron, which is helpful for diagnosing anemia. The usual range for men is 18-270 ng/mL and the range for women is 18-160 ng/mL.
252:
Ferritin is a highly conserved protein through all domains of life. It is so conserved that subunits from horses and humans can assemble together into a functional protein. Each subunit is composed of five alpha helices.
1019:
Kuwata H, Yip TT, Yip CL, Tomita M, Hutchens TW (April 1998). "Bactericidal domain of lactoferrin: detection, quantitation, and characterization of lactoferricin in serum by SELDI affinity mass spectrometry".
446:
Solomon EI, Brunold TC, Davis MI, Kemsley JN, Lee SK, Lehnert N, Neese F, Skulan AJ, Yang YS, Zhou J (January 2000). "Geometric and electronic structure/function correlations in non-heme iron enzymes".
177:
Transferrin is found in human plasma, and it is used to traffic and import non-heme iron. It travels freely in the extracellular space. When its iron is needed by the cell, it is brought into the
106:
production and aqueous formation of carbonic acid in respiring cells, oxygenated hemoglobin dissociates in order to deliver the necessary oxygen to the cells. Hemoglobin has a binding affinity for
900:
Mazurier J, Spik G (May 1980). "Comparative study of the iron-binding properties of human transferrins. I. Complete and sequential iron saturation and desaturation of the lactotransferrin".
102:
during pregnancy. Hemoglobin has a lower oxygen affinity at low pH. This allows for rapid dissociation as oxygenated hemoglobin is transported to cells throughout the body. Because of the CO
736:
Johnson, Deborah C.; Dean, Dennis R.; Smith, Archer D.; Johnson, Michael K. (Feb 18, 2005). "Structure, Function, and
Formation of Biological Iron-Sulfur Clusters".
658:
Hasselbalch KA (December 1964). "Calculation Of The
Hydrogen Ion Concentration Of Blood From Free And Bound Carbon Dioxide Oxygen Binding As A Function Of Ph".
722:
54:
The heme group uses four equatorial ligands in the porphyrin ring, with the two axial ligands being the histidine side chain and molecular oxygen.
1595:
1496:
1481:
1279:
1190:
188:
Transferrin has a molecular weight of about 80 kDa. It is a glycoprotein, meaning that it has sugars attached to its amino acid chain.
346:
De Sousa M, Breedvelt F, Dynesius-Trentham R, Trentham D, Lum J (1988). "Iron, iron-binding proteins and immune system cells".
541:. Gaithersburg, MD: Analytical Coordination Chemistry Section Analytical Chemistry Division Institute for Materials Research.
1229:
218:
Lactoferrin has significant antimicrobial properties. It is found in the highest concentration of 150 ng/mL in human
1588:
1486:
1527:
1532:
1547:
1581:
1274:
1183:
698:
634:
984:
Farnaud S, Evans RW (November 2003). "Lactoferrin--a multifunctional protein with antimicrobial properties".
68:
484:"The function and catalysis of 2-oxoglutarate-dependent oxygenases involved in plant flavonoid biosynthesis"
1573:
1344:
784:
Aisen P, Enns C, Wessling-Resnick M (October 2001). "Chemistry and biology of eukaryotic iron metabolism".
1389:
1557:
1358:
1211:
1456:
1176:
111:
693:. Nelson, David L. (David Lee), 1942-, Cox, Michael M. (3rd. ed.). New York: Worth Publishers.
1700:
1348:
282:
16:
Carrier proteins and metalloproteins that are important in iron metabolism and the immune response.
1669:
1562:
1436:
1384:
278:
138:
1552:
58:
Heme proteins are proteins that contain a heme prosthetic group. The heme group consists of a
42:. Notable enzymes include tryptophan dioxygenase, ferredoxin, and 2-oxoglutarate dioxygenase.
1612:
749:
153:
1148:
89:
A visual depiction of the conformational change undergone by hemoglobin upon oxygen binding.
1679:
1522:
1418:
575:
355:
182:
8:
1234:
206:
869:
844:
579:
359:
1664:
1473:
1125:
1108:
1084:
1059:
961:
936:
882:
716:
539:
Analytical
Coordination Chemistry Section: Summary of Activities July 1967 to June 1968
510:
483:
423:
398:
379:
367:
328:
223:
997:
797:
1130:
1089:
1037:
1001:
966:
917:
913:
886:
874:
801:
761:
753:
704:
694:
687:
671:
640:
630:
623:
603:
598:
563:
515:
464:
428:
371:
320:
238:
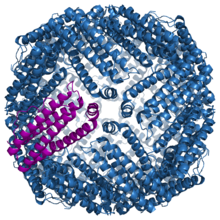
Protein structure of fully assembled ferritin. A single subunit is colored in purple.
383:
332:
1631:
1491:
1120:
1079:
1071:
1029:
993:
956:
948:
909:
864:
856:
793:
745:
667:
593:
583:
542:
505:
495:
456:
418:
410:
363:
312:
1604:
1340:
1265:
1203:
1075:
107:
23:
316:
1608:
1463:
1331:
820:"TF - Serotransferrin precursor - Homo sapiens (Human) - TF gene & protein"
568:
Proceedings of the
National Academy of Sciences of the United States of America
27:
860:
414:
1694:
1537:
1401:
1363:
1326:
1168:
757:
1603:
708:
644:
1674:
1321:
1093:
1033:
1005:
878:
805:
765:
607:
588:
519:
468:
432:
85:
1134:
1041:
970:
921:
375:
345:
324:
1659:
1643:
1638:
1501:
1353:
1269:
952:
819:
500:
197:
172:
547:
534:
123:
80:
31:
460:
163:
1542:
1506:
845:"Molecular structure, binding properties and dynamics of lactoferrin"
219:
97:
Hemoglobin has various affinities, depending on pH, structure, and CO
59:
1626:
243:
210:
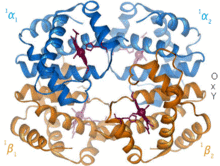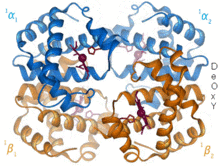
Depiction of lactoferrin (left) competitively binding iron over an
1199:
178:
110:
that is 250 times greater than for oxygen. This is the basis of
1314:
1309:
1304:
1299:
1294:
1289:
1284:
1249:
1244:
1239:
1224:
1219:
786:
1451:
1446:
1441:
1106:
266:
234:
50:
1149:"What Is a Ferritin Blood Test? What Do the Results Mean?"
445:
783:
564:"A signature of the T → R transition in human hemoglobin"
114:, as hemoglobin can no longer transport oxygen to cells.
735:
34:
and the immune response. Iron is required for life.
1022:
399:"The essential nature of iron usage and regulation"
167:
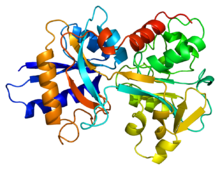
Structure visualization of human serum transferrin.
38:
hydroxyl group addition of one or both atoms from o
1018:
686:
622:
620:
1060:"Forging a field: the golden age of iron biology"
934:
1692:
482:Cheng AX, Han XJ, Wu YF, Lou HX (January 2014).
1107:Crichton RR, Charloteaux-Wauters M (May 1987).
561:
1198:
1589:
1184:
481:
983:
977:
899:
836:
721:: CS1 maint: multiple names: authors list (
1012:
928:
893:
657:
555:
488:International Journal of Molecular Sciences
475:
439:
1596:
1582:
1280:Insulin-like growth factor binding protein
1191:
1177:
842:
396:
348:Annals of the New York Academy of Sciences
303:Brock JH (1989). "Iron-binding proteins".
1124:
1083:
960:
935:Sánchez L, Calvo M, Brock JH (May 1992).
868:
597:
587:
546:
509:
499:
422:
305:Acta Paediatrica Scandinavica. Supplement
281:at the U.S. National Library of Medicine
750:10.1146/annurev.biochem.74.082803.133518
629:(5th ed.). New York: W.H. Freeman.
233:
205:
162:
84:
49:
1057:
621:Berg JM, Tymoczko JL, Stryer L (2002).
132:
1693:
562:Mihailescu MR, Russu IM (March 2001).
1577:
1172:
1053:
1051:
779:
777:
775:
532:
302:
849:Cellular and Molecular Life Sciences
843:Baker EN, Baker HM (November 2005).
689:Lehninger principles of biochemistry
147:
13:
1126:10.1111/j.1432-1033.1987.tb11155.x
1048:
812:
772:
684:
368:10.1111/j.1749-6632.1988.tb55515.x
14:
1712:
1533:Cholesterylester transfer protein
397:Kaplan J, Ward DM (August 2013).
272:
1113:European Journal of Biochemistry
941:Archives of Disease in Childhood
937:"Biological role of lactoferrin"
672:10.1097/00132586-196412000-00059
45:
1548:Latent TGF-beta binding protein
1497:Photosynthetic Reaction Centers
1141:
1100:
729:
678:
1482:Plant Light-Harvesting Complex
1275:Growth hormone binding protein
685:L., Lehninger, Albert (2000).
651:
614:
526:
390:
339:
296:
191:
158:
117:
1:
998:10.1016/S0161-5890(03)00152-4
902:Biochimica et Biophysica Acta
798:10.1016/s1357-2725(01)00063-2
738:Annual Review of Biochemistry
289:
74:
1345:Sex hormone binding globulin
1109:"Iron transport and storage"
1076:10.1182/blood-2007-12-077388
914:10.1016/0304-4165(80)90112-9
7:
1390:Calmodulin-binding proteins
317:10.1111/apa.1989.78.s361.31
260:
229:
30:that are important in iron
10:
1717:
1558:Membrane transport protein
1359:Thyroxine-binding globulin
241:
195:
170:
151:
136:
121:
78:
1652:
1619:
1515:
1487:Orange Carotenoid Protein
1472:
1429:
1372:
1258:
1210:
861:10.1007/s00018-005-5368-9
415:10.1016/j.cub.2013.05.033
112:carbon monoxide poisoning
1349:Androgen binding protein
1058:Andrews NC (July 2008).
660:Survey of Anesthesiology
283:Medical Subject Headings
1563:Odorant binding protein
1437:Retinol binding protein
1385:Calcium-binding protein
1553:Major urinary proteins
1034:10.1006/bbrc.1998.8466
589:10.1073/pnas.071493598
239:
215:
168:
90:
55:
1613:Non-heme iron protein
1414:Iron-binding proteins
533:Menis, Oscar (1968).
279:Iron-binding+proteins
237:
209:
166:
154:Non-heme iron protein
88:
67:molecule, forming an
53:
20:Iron-binding proteins
1680:Tyrosine hydroxylase
1523:Acyl carrier protein
1419:Transferrin receptor
986:Molecular Immunology
953:10.1136/adc.67.5.657
535:"Technical Note 454"
501:10.3390/ijms15011080
214:siderophore (right).
183:transferrin receptor
133:Iron-sulfur proteins
1670:Iron–sulfur protein
1543:GTP-binding protein
580:2001PNAS...98.3773M
360:1988NYASA.526..310S
139:Iron–sulfur protein
63:chain and a bound O
1665:Inositol oxygenase
548:10.6028/nbs.tn.454
240:
224:lipopolysaccharide
216:
169:
91:
56:
1688:
1687:
1571:
1570:
461:10.1021/cr9900275
148:Non-heme proteins
1708:
1632:Bacterioferritin
1605:Carrier proteins
1598:
1591:
1584:
1575:
1574:
1492:Phycobiliprotein
1204:carrier proteins
1193:
1186:
1179:
1170:
1169:
1163:
1162:
1160:
1159:
1145:
1139:
1138:
1128:
1104:
1098:
1097:
1087:
1055:
1046:
1045:
1016:
1010:
1009:
981:
975:
974:
964:
932:
926:
925:
897:
891:
890:
872:
840:
834:
833:
831:
830:
816:
810:
809:
781:
770:
769:
733:
727:
726:
720:
712:
692:
682:
676:
675:
655:
649:
648:
628:
618:
612:
611:
601:
591:
559:
553:
552:
550:
530:
524:
523:
513:
503:
479:
473:
472:
449:Chemical Reviews
443:
437:
436:
426:
394:
388:
387:
343:
337:
336:
300:
24:carrier proteins
1716:
1715:
1711:
1710:
1709:
1707:
1706:
1705:
1701:Iron metabolism
1691:
1690:
1689:
1684:
1648:
1615:
1609:metalloproteins
1602:
1572:
1567:
1528:Adaptor protein
1511:
1468:
1425:
1368:
1341:steroid hormone
1266:peptide hormone
1254:
1206:
1197:
1167:
1166:
1157:
1155:
1147:
1146:
1142:
1105:
1101:
1056:
1049:
1017:
1013:
982:
978:
933:
929:
898:
894:
841:
837:
828:
826:
824:www.uniprot.org
818:
817:
813:
782:
773:
734:
730:
714:
713:
701:
683:
679:
656:
652:
637:
619:
615:
560:
556:
531:
527:
480:
476:
444:
440:
403:Current Biology
395:
391:
344:
340:
301:
297:
292:
275:
263:
246:
232:
200:
194:
175:
161:
156:
150:
141:
135:
126:
120:
108:carbon monoxide
105:
100:
83:
77:
66:
48:
41:
28:metalloproteins
17:
12:
11:
5:
1714:
1704:
1703:
1686:
1685:
1683:
1682:
1677:
1672:
1667:
1662:
1656:
1654:
1650:
1649:
1647:
1646:
1641:
1636:
1635:
1634:
1623:
1621:
1617:
1616:
1601:
1600:
1593:
1586:
1578:
1569:
1568:
1566:
1565:
1560:
1555:
1550:
1545:
1540:
1535:
1530:
1525:
1519:
1517:
1513:
1512:
1510:
1509:
1504:
1499:
1494:
1489:
1484:
1478:
1476:
1470:
1469:
1467:
1466:
1464:Transcobalamin
1461:
1460:
1459:
1454:
1449:
1444:
1433:
1431:
1427:
1426:
1424:
1423:
1422:
1421:
1416:
1406:
1405:
1404:
1394:
1393:
1392:
1387:
1376:
1374:
1370:
1369:
1367:
1366:
1361:
1356:
1351:
1337:
1336:
1335:
1334:
1329:
1319:
1318:
1317:
1312:
1307:
1302:
1297:
1292:
1287:
1277:
1272:
1262:
1260:
1256:
1255:
1253:
1252:
1247:
1242:
1237:
1232:
1227:
1222:
1216:
1214:
1208:
1207:
1196:
1195:
1188:
1181:
1173:
1165:
1164:
1140:
1119:(3): 485–506.
1099:
1047:
1011:
992:(7): 395–405.
976:
927:
908:(2): 399–408.
892:
855:(22): 2531–9.
835:
811:
792:(10): 940–59.
771:
744:(1): 247–281.
728:
699:
677:
650:
635:
613:
554:
525:
494:(1): 1080–95.
474:
455:(1): 235–350.
438:
409:(15): R642-6.
389:
338:
294:
293:
291:
288:
287:
286:
274:
273:External links
271:
270:
269:
262:
259:
242:Main article:
231:
228:
196:Main article:
193:
190:
171:Main article:
160:
157:
152:Main article:
149:
146:
137:Main article:
134:
131:
122:Main article:
119:
116:
103:
98:
79:Main article:
76:
73:
64:
47:
44:
39:
15:
9:
6:
4:
3:
2:
1713:
1702:
1699:
1698:
1696:
1681:
1678:
1676:
1673:
1671:
1668:
1666:
1663:
1661:
1658:
1657:
1655:
1651:
1645:
1642:
1640:
1637:
1633:
1630:
1629:
1628:
1625:
1624:
1622:
1618:
1614:
1610:
1606:
1599:
1594:
1592:
1587:
1585:
1580:
1579:
1576:
1564:
1561:
1559:
1556:
1554:
1551:
1549:
1546:
1544:
1541:
1539:
1538:F-box protein
1536:
1534:
1531:
1529:
1526:
1524:
1521:
1520:
1518:
1514:
1508:
1505:
1503:
1500:
1498:
1495:
1493:
1490:
1488:
1485:
1483:
1480:
1479:
1477:
1475:
1471:
1465:
1462:
1458:
1455:
1453:
1450:
1448:
1445:
1443:
1440:
1439:
1438:
1435:
1434:
1432:
1428:
1420:
1417:
1415:
1412:
1411:
1410:
1407:
1403:
1402:Ceruloplasmin
1400:
1399:
1398:
1395:
1391:
1388:
1386:
1383:
1382:
1381:
1378:
1377:
1375:
1373:Metal/element
1371:
1365:
1364:Transthyretin
1362:
1360:
1357:
1355:
1352:
1350:
1346:
1342:
1339:
1338:
1333:
1330:
1328:
1327:Neurophysin I
1325:
1324:
1323:
1320:
1316:
1313:
1311:
1308:
1306:
1303:
1301:
1298:
1296:
1293:
1291:
1288:
1286:
1283:
1282:
1281:
1278:
1276:
1273:
1271:
1267:
1264:
1263:
1261:
1257:
1251:
1248:
1246:
1243:
1241:
1238:
1236:
1233:
1231:
1228:
1226:
1223:
1221:
1218:
1217:
1215:
1213:
1209:
1205:
1201:
1194:
1189:
1187:
1182:
1180:
1175:
1174:
1171:
1154:
1150:
1144:
1136:
1132:
1127:
1122:
1118:
1114:
1110:
1103:
1095:
1091:
1086:
1081:
1077:
1073:
1070:(2): 219–30.
1069:
1065:
1061:
1054:
1052:
1043:
1039:
1035:
1031:
1028:(3): 764–73.
1027:
1023:
1015:
1007:
1003:
999:
995:
991:
987:
980:
972:
968:
963:
958:
954:
950:
947:(5): 657–61.
946:
942:
938:
931:
923:
919:
915:
911:
907:
903:
896:
888:
884:
880:
876:
871:
866:
862:
858:
854:
850:
846:
839:
825:
821:
815:
807:
803:
799:
795:
791:
787:
780:
778:
776:
767:
763:
759:
755:
751:
747:
743:
739:
732:
724:
718:
710:
706:
702:
696:
691:
690:
681:
673:
669:
666:(6): 607–32.
665:
661:
654:
646:
642:
638:
632:
627:
626:
617:
609:
605:
600:
595:
590:
585:
581:
577:
574:(7): 3773–7.
573:
569:
565:
558:
549:
544:
540:
536:
529:
521:
517:
512:
507:
502:
497:
493:
489:
485:
478:
470:
466:
462:
458:
454:
450:
442:
434:
430:
425:
420:
416:
412:
408:
404:
400:
393:
385:
381:
377:
373:
369:
365:
361:
357:
354:(1): 310–22.
353:
349:
342:
334:
330:
326:
322:
318:
314:
310:
306:
299:
295:
284:
280:
277:
276:
268:
265:
264:
258:
254:
250:
245:
236:
227:
225:
221:
213:
208:
204:
199:
189:
186:
184:
180:
174:
165:
155:
145:
140:
130:
125:
115:
113:
109:
95:
87:
82:
72:
70:
61:
52:
46:Heme proteins
43:
35:
33:
29:
25:
21:
1675:Lipoxygenase
1413:
1408:
1396:
1379:
1322:Neurophysins
1156:. Retrieved
1152:
1143:
1116:
1112:
1102:
1067:
1063:
1025:
1021:
1014:
989:
985:
979:
944:
940:
930:
905:
901:
895:
852:
848:
838:
827:. Retrieved
823:
814:
789:
785:
741:
737:
731:
688:
680:
663:
659:
653:
625:Biochemistry
624:
616:
571:
567:
557:
538:
528:
491:
487:
477:
452:
448:
441:
406:
402:
392:
351:
347:
341:
308:
304:
298:
255:
251:
247:
217:
211:
201:
187:
176:
142:
127:
96:
92:
57:
36:
19:
18:
1660:Hemerythrin
1644:Transferrin
1639:Lactoferrin
1502:Phytochrome
1354:Transcortin
1270:Follistatin
198:Lactoferrin
192:Lactoferrin
173:Transferrin
159:Transferrin
118:Cytochromes
1212:Fatty acid
1158:2018-11-11
829:2018-11-11
700:1572591536
636:0716730510
290:References
124:Cytochrome
81:Hemoglobin
75:Hemoglobin
69:octahedral
32:metabolism
1507:Rhodopsin
887:218464085
758:0066-4154
717:cite book
311:: 31–43.
220:colostrum
71:complex.
60:porphyrin
1695:Category
1627:Ferritin
1200:Proteins
1094:18606887
1006:14568385
879:16261257
870:11139133
806:11470229
766:15952888
709:42619569
645:48055706
608:11259676
520:24434621
469:11749238
433:23928078
384:12756539
333:44752615
261:See also
244:Ferritin
230:Ferritin
1653:nonheme
1474:Pigment
1430:Vitamin
1380:calcium
1259:Hormone
1135:3032619
1085:2442739
1042:9588189
971:1599309
962:1793702
922:6770907
576:Bibcode
511:3907857
424:3928970
376:3291685
356:Bibcode
325:2485582
212:E. coli
179:cytosol
1397:copper
1315:IGFBP7
1310:IGFBP6
1305:IGFBP5
1300:IGFBP4
1295:IGFBP3
1290:IGFBP2
1285:IGFBP1
1133:
1092:
1082:
1040:
1004:
969:
959:
920:
885:
877:
867:
804:
764:
756:
707:
697:
643:
633:
606:
596:
518:
508:
467:
431:
421:
382:
374:
331:
323:
285:(MeSH)
1516:Other
1250:FABP7
1245:FABP6
1240:FABP5
1235:FABP4
1230:FABP3
1225:FABP2
1220:FABP1
1153:WebMD
1064:Blood
883:S2CID
599:31128
380:S2CID
329:S2CID
181:by a
1620:heme
1409:iron
1131:PMID
1090:PMID
1038:PMID
1002:PMID
967:PMID
918:PMID
875:PMID
802:PMID
762:PMID
754:ISSN
723:link
705:OCLC
695:ISBN
641:OCLC
631:ISBN
604:PMID
516:PMID
465:PMID
429:PMID
372:PMID
321:PMID
267:Iron
26:and
22:are
1121:doi
1117:164
1080:PMC
1072:doi
1068:112
1030:doi
1026:245
994:doi
957:PMC
949:doi
910:doi
906:629
865:PMC
857:doi
794:doi
746:doi
668:doi
594:PMC
584:doi
543:doi
506:PMC
496:doi
457:doi
453:100
419:PMC
411:doi
364:doi
352:526
313:doi
309:361
1697::
1611::
1607:,
1343::
1332:II
1268::
1202::
1151:.
1129:.
1115:.
1111:.
1088:.
1078:.
1066:.
1062:.
1050:^
1036:.
1024:.
1000:.
990:40
988:.
965:.
955:.
945:67
943:.
939:.
916:.
904:.
881:.
873:.
863:.
853:62
851:.
847:.
822:.
800:.
790:33
788:.
774:^
760:.
752:.
742:74
740:.
719:}}
715:{{
703:.
662:.
639:.
602:.
592:.
582:.
572:98
570:.
566:.
537:.
514:.
504:.
492:15
490:.
486:.
463:.
451:.
427:.
417:.
407:23
405:.
401:.
378:.
370:.
362:.
350:.
327:.
319:.
307:.
1597:e
1590:t
1583:v
1457:4
1452:3
1447:2
1442:1
1347:/
1192:e
1185:t
1178:v
1161:.
1137:.
1123::
1096:.
1074::
1044:.
1032::
1008:.
996::
973:.
951::
924:.
912::
889:.
859::
832:.
808:.
796::
768:.
748::
725:)
711:.
674:.
670::
664:8
647:.
610:.
586::
578::
551:.
545::
522:.
498::
471:.
459::
435:.
413::
386:.
366::
358::
335:.
315::
104:2
99:2
65:2
40:2
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.