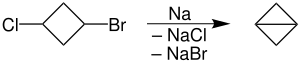409:
383:
594:
628:
362:
117:
256:
experienced at the transition state. Although three-membered rings are more strained, formation of aziridine is faster than formation of azetidine due to the proximity of the leaving group and nucleophile in the former, which increases the probability that they would meet in a reactive conformation.
66:
Intramolecular reactions, especially ones leading to the formation of 5- and 6-membered rings, are rapid compared to an analogous intermolecular process. This is largely a consequence of the reduced entropic cost for reaching the transition state of ring formation and the absence of significant
585:) which are fairly inert in many organic reactions yet can be cleaved by specific reagents. The main hurdle for this strategy to work is selecting the proper length for the tether and making sure reactive groups have an optimal orientation with respect to each other. An examples is a
545:
526:
810:
Coates, R. M.; Senter, P. D.; Baker, W. R. (1982). "Annelative Ring
Expansion via Intramolecular Photocycloaddition of α,β-Unsaturated γ-Lactones and Reductive Cleavage: Synthesis of Hydrocyclopentacyclooctene-5-carboxylates".
467:
67:
strain associated with formation of rings of these sizes. For the formation of different ring sizes via cyclization of substrates of varying tether length, the order of reaction rates (rate constants
296:
Many reactions in organic chemistry can occur in either an intramolecular or intermolecular senses. Some reactions are by definition intramolecular or are only practiced intramolecularly, e.g.,
288:
Although the details may change somewhat, the general trends hold for a variety of intramolecular reactions, including radical-mediated and (in some cases) transition metal-catalyzed processes.
838:
Tamura, Y.; Kita, Y.; Ishibashi, H.; Ikeda, M. (1971). "Intramolecular photocycloaddition of 3-allyloxy- and 3-allylamino-cyclohex-2-enones: formation of oxa- and aza-bicyclohexanes".
376:, involving reductive coupling of alkyl halides, essentially is only useful when conducted intramolecularly. Its utility is illustrated with the synthesis of strained rings:
496:
520:
422:
Many tools and concepts have been developed to exploit the advantages of intramolecular cyclizations. For example, installing large substituents exploits the
1037:
Booker-Milburn, Kevin I.; Gulten, Sirin; Sharpe, Andrew (1997). "Diastereoselective intramolecular photochemical cycloaddition reactions of tethered l-(+)-
285:), the rate constants level off, as the distance between the leaving group and nucleophile is now so large the reaction is now effectively intermolecular.
395:
of diesters almost uniquely produces 10-membered carbocycles, which are difficult to construct otherwise. Another example is the 2+2 cycloaddition of
113:
as shown below for a series of ω-bromoalkylamines. This somewhat complicated rate trend reflects the interplay of these entropic and strain factors:
1087:
916:
Cox, Liam R.; Ley, Steven V. (2007). "Use of the
Temporary Connection in Organic Synthesis". In Diederich, François; Stang, Peter J. (eds.).
499:
544:
525:
478:
of the reaction. Longer tethers tend to generate the "straight" product where the terminal carbon of the alkene is linked to the
949:
Bracegirdle, S.; Anderson, E. A. (2010). "Recent advances in the use of temporary silicon tethers in metal-mediated reactions".
273:) is particularly disfavorable due to a combination of an increasingly unfavorable entropic cost and the additional presence of
990:
795:
1299:
1176:
1133:
599:
In this particular reaction, the tether angle bringing the reactive groups together is effectively reduced by placing
1440:
1080:
933:
664:
627:
593:
840:
1348:
1343:
1153:
691:
361:
659:. New Delhi: Medtech (Scientific International, reprint of 1998 revised 4th edition, Macmillan). p. 198.
607:. No reaction takes place when these bulky groups are replaced by smaller methyl groups. Another example is a
1513:
1508:
1534:
1073:
349:
785:
561:
Otherwise-intermolecular reactions can be made temporarily intramolecular by linking both reactants by a
1544:
1539:
1478:
1168:
466:
51:, two reaction sites are contained within a single molecule. This configuration elevates the effective
1205:
1105:
586:
533:
Tethered reactions have been used to synthesize organic compounds with interesting ring systems and
1435:
604:
1483:
460:
681:
408:
1238:
427:
300:
1468:
1400:
1258:
1248:
481:
505:
430:
suppress intermolecular processes. One set of tools involves tethering as discussed below.
1463:
1191:
423:
392:
319:
8:
1473:
1405:
1390:
1333:
715:
Michael C. Willis (2009). "Transition Metal
Catalyzed Alkene and Alkyne Hydroacylation".
382:
274:
1498:
1268:
1097:
1014:
985:
893:
868:
537:. For example, photocyclization was used to construct the tricyclic core structure in
502:. When the tether consists only two carbons, the “bent” product is generated where the
310:
304:
32:
391:
Some transformations that are enabled or enhanced intramolecularly. For example, the
1493:
1488:
1450:
1395:
1314:
1294:
1230:
1019:
966:
929:
898:
791:
753:
733:
697:
687:
660:
1425:
1374:
1328:
1051:
1009:
999:
958:
921:
888:
880:
849:
820:
762:
725:
614:
with two alkene groups tethered through a silicon acetal group (racemic, the other
48:
28:
1503:
1415:
1364:
566:
475:
1210:
1199:
866:
373:
353:
324:
60:
565:
with all the advantages associated to it. Popular choices of tether contain a
1528:
1458:
1430:
1338:
1289:
1263:
1043:
867:
Corey, E. J.; Kang, M. C.; Desai, M. C.; Ghosh, A. K.; Houpis, I. N. (1988).
766:
611:
608:
570:
456:
400:
396:
56:
52:
1065:
701:
1410:
1216:
1123:
1113:
1023:
1004:
970:
902:
737:
578:
925:
1369:
1304:
853:
781:
574:
452:
253:
884:
824:
655:
Streitwieser, Andrew; Heathcock, Clayton H.; Kosower, Edward M. (2017).
522:-carbon of the enone is connected to the terminal carbon of the alkene.
962:
717:
635:
615:
538:
534:
39:, a property or phenomenon limited to the extent of a single molecule.
16:
Process or characteristic limited to the structure of a single molecule
809:
729:
1420:
1055:
600:
20:
116:
327:
is almost invariably practiced intramolecularly to produce ketones.
36:
1038:
986:"The use of silicon-based tethers for the Pauson-Khand reaction"
787:
Advanced
Organic Chemistry: Reactions, Mechanisms, and Structure
589:
of an alkene and an alkyne tethered together via a silyl ether.
562:
529:
Effects of the length of tether on photocyclization reaction
314:
277:
arising from steric interactions across the ring. Finally, for
751:
Lampman, Gary M.; Aumiller, James C. (1971). "Bicyclobutane".
1323:
779:
790:(6th ed.), New York: Wiley-Interscience, p. 1461,
654:
619:
451:
Tethered intramolecular reactions entail the formation of
1036:
434:
1143:
837:
463:. Tethering ensures formation of a multi-cyclic system.
59:. Many intramolecular reactions are observed where the
983:
508:
484:
433:
948:
686:. Oxford: Oxford University Press. pp. 454].
514:
490:
714:
1526:
679:
618:not depicted), which is subsequently cleaved by
750:
984:Dobbs, A.; Miller, I.; Martinovic, S. (2007).
548:Tethered reaction in the total synthesis of (
1095:
1081:
303:of diesters is the intramolecular version of
115:
55:of the reacting partners resulting in high
1088:
1074:
1013:
1003:
892:
124:Relative rate constants for cyclization (
869:"Total Synthesis of (.+-.)-Ginkgolide B"
1527:
1114:Unimolecular nucleophilic substitution
991:Beilstein Journal of Organic Chemistry
915:
252:), the slow rates is a consequence of
1124:Bimolecular nucleophilic substitution
1069:
708:
474:The length of the tether affects the
417:
31:or characteristic limited within the
603:groups on the silicon atom via the
556:
1177:Electrophilic aromatic substitution
13:
1144:Nucleophilic internal substitution
1134:Nucleophilic aromatic substitution
543:
524:
470:Tethered intramolecular reactions
465:
435:Tethered intramolecular reactions
407:
381:
360:
14:
1556:
1041:derived tetrahydrophthalimides".
657:Introduction to Organic Chemistry
257:The same reasoning holds for the
42:
626:
592:
365:The Nazarov cyclization reaction
1300:Lindemann–Hinshelwood mechanism
1030:
977:
942:
1349:Outer sphere electron transfer
1344:Inner sphere electron transfer
1154:Nucleophilic acyl substitution
909:
860:
831:
803:
773:
744:
673:
648:
1:
1514:Diffusion-controlled reaction
641:
63:version does not take place.
350:Nazarov cyclization reaction
7:
1169:Electrophilic substitutions
918:Templated Organic Synthesis
291:
78:-membered ring) is usually
10:
1561:
1479:Energy profile (chemistry)
1441:More O'Ferrall–Jencks plot
1106:Nucleophilic substitutions
1509:Michaelis–Menten kinetics
1449:
1383:
1357:
1313:
1277:
1229:
1190:
1167:
1104:
680:Jonathan Clayden (2001).
1436:Potential energy surface
1315:Electron/Proton transfer
1200:Unimolecular elimination
767:10.15227/orgsyn.051.0055
634:Without the tether, the
622:yielding the endo-diol.
74:for the formation of an
1484:Transition state theory
1285:Intramolecular reaction
1211:Bimolecular elimination
491:{\displaystyle \alpha }
461:2+2 photocycloadditions
428:High dilution reactions
1278:Unimolecular reactions
1239:Electrophilic addition
1005:10.1186/1860-5397-3-21
553:
530:
516:
515:{\displaystyle \beta }
492:
476:stereochemical outcome
471:
412:
386:
366:
301:Dieckmann condensation
263:5-, 6-, and 7-membered
120:
1469:Rate-determining step
1401:Reactive intermediate
1259:Free-radical addition
1249:Nucleophilic addition
1192:Elimination reactions
926:10.1002/9783527613526
587:Pauson–Khand reaction
547:
528:
517:
493:
469:
411:
385:
364:
352:for the synthesis of
283:14-membered or higher
119:
1464:Equilibrium constant
920:. pp. 274–395.
854:10.1039/C29710001167
605:Thorpe–Ingold effect
506:
482:
424:Thorpe-Ingold effect
393:acyloin condensation
320:Smiles rearrangement
267:'medium-sized rings'
265:). The formation of
1535:Reaction mechanisms
1474:Reaction coordinate
1406:Radical (chemistry)
1391:Elementary reaction
1334:Grotthuss mechanism
1098:reaction mechanisms
885:10.1021/ja00210a083
825:10.1021/jo00140a001
780:Smith, Michael B.;
459:via intramolecular
336:=CHR' → RC(O)CH
275:transannular strain
138:
136:
1499:Arrhenius equation
1269:Oxidative addition
1231:Addition reactions
963:10.1039/C0CS00007H
554:
531:
512:
488:
472:
418:Tools and concepts
413:
387:
367:
311:Madelung synthesis
305:aldol condensation
259:'unstrained rings'
250:3- and 4- membered
137:
123:
121:
47:In intramolecular
1545:Organic chemistry
1540:Molecular physics
1522:
1521:
1494:Activated complex
1489:Activation energy
1451:Chemical kinetics
1396:Reaction dynamics
1295:Photodissociation
1050:(15): 1385–1386.
957:(11): 4114–4129.
797:978-0-471-72091-1
754:Organic Syntheses
730:10.1021/cr900096x
683:Organic chemistry
557:Molecular tethers
271:8- to 13-membered
242:
241:
49:organic reactions
1552:
1426:Collision theory
1375:Matrix isolation
1329:Harpoon reaction
1206:E1cB-elimination
1090:
1083:
1076:
1067:
1066:
1060:
1059:
1056:10.1039/a702386c
1034:
1028:
1027:
1017:
1007:
981:
975:
974:
946:
940:
939:
913:
907:
906:
896:
873:J. Am. Chem. Soc
864:
858:
857:
835:
829:
828:
807:
801:
800:
777:
771:
769:
748:
742:
741:
712:
706:
705:
677:
671:
670:
652:
630:
596:
521:
519:
518:
513:
497:
495:
494:
489:
448:
447:
443:
436:
139:
122:
1560:
1559:
1555:
1554:
1553:
1551:
1550:
1549:
1525:
1524:
1523:
1518:
1504:Eyring equation
1445:
1416:Stereochemistry
1379:
1365:Solvent effects
1353:
1309:
1273:
1254:
1244:
1225:
1220:
1186:
1182:
1163:
1159:
1149:
1139:
1129:
1119:
1100:
1094:
1064:
1063:
1035:
1031:
982:
978:
947:
943:
936:
914:
910:
865:
861:
841:J. Chem. Soc. D
836:
832:
808:
804:
798:
778:
774:
749:
745:
713:
709:
694:
678:
674:
667:
653:
649:
644:
583:silicon tethers
567:carbonate ester
559:
507:
504:
503:
498:-carbon of the
483:
480:
479:
449:
445:
441:
440:reactions": -->
439:
438:
420:
354:cyclopentenones
343:
339:
335:
294:
178:
165:
152:
134:
112:
105:
98:
91:
84:
72:
45:
17:
12:
11:
5:
1558:
1548:
1547:
1542:
1537:
1520:
1519:
1517:
1516:
1511:
1506:
1501:
1496:
1491:
1486:
1481:
1476:
1471:
1466:
1461:
1455:
1453:
1447:
1446:
1444:
1443:
1438:
1433:
1428:
1423:
1418:
1413:
1408:
1403:
1398:
1393:
1387:
1385:
1384:Related topics
1381:
1380:
1378:
1377:
1372:
1367:
1361:
1359:
1358:Medium effects
1355:
1354:
1352:
1351:
1346:
1341:
1336:
1331:
1326:
1320:
1318:
1311:
1310:
1308:
1307:
1302:
1297:
1292:
1287:
1281:
1279:
1275:
1274:
1272:
1271:
1266:
1261:
1256:
1252:
1246:
1242:
1235:
1233:
1227:
1226:
1224:
1223:
1218:
1214:
1208:
1203:
1196:
1194:
1188:
1187:
1185:
1184:
1180:
1173:
1171:
1165:
1164:
1162:
1161:
1157:
1151:
1147:
1141:
1137:
1131:
1127:
1121:
1117:
1110:
1108:
1102:
1101:
1093:
1092:
1085:
1078:
1070:
1062:
1061:
1029:
976:
951:Chem. Soc. Rev
941:
934:
908:
879:(2): 649–651.
859:
830:
802:
796:
772:
743:
724:(2): 725–748.
707:
692:
672:
665:
646:
645:
643:
640:
632:
631:
558:
555:
552:- Ginkgolide B
511:
487:
437:
432:
419:
416:
415:
414:
389:
388:
378:
377:
374:Wurtz reaction
369:
368:
357:
356:
346:
345:
341:
337:
333:
329:
328:
325:Hydroacylation
322:
317:
308:
293:
290:
240:
239:
236:
233:
230:
227:
224:
220:
219:
216:
213:
210:
207:
204:
200:
199:
196:
193:
190:
187:
184:
180:
179:
176:
171:
166:
163:
158:
153:
150:
145:
132:
110:
103:
96:
89:
82:
70:
61:intermolecular
57:reaction rates
44:
43:Relative rates
41:
25:intramolecular
15:
9:
6:
4:
3:
2:
1557:
1546:
1543:
1541:
1538:
1536:
1533:
1532:
1530:
1515:
1512:
1510:
1507:
1505:
1502:
1500:
1497:
1495:
1492:
1490:
1487:
1485:
1482:
1480:
1477:
1475:
1472:
1470:
1467:
1465:
1462:
1460:
1459:Rate equation
1457:
1456:
1454:
1452:
1448:
1442:
1439:
1437:
1434:
1432:
1431:Arrow pushing
1429:
1427:
1424:
1422:
1419:
1417:
1414:
1412:
1409:
1407:
1404:
1402:
1399:
1397:
1394:
1392:
1389:
1388:
1386:
1382:
1376:
1373:
1371:
1368:
1366:
1363:
1362:
1360:
1356:
1350:
1347:
1345:
1342:
1340:
1339:Marcus theory
1337:
1335:
1332:
1330:
1327:
1325:
1322:
1321:
1319:
1316:
1312:
1306:
1303:
1301:
1298:
1296:
1293:
1291:
1290:Isomerization
1288:
1286:
1283:
1282:
1280:
1276:
1270:
1267:
1265:
1264:Cycloaddition
1262:
1260:
1257:
1250:
1247:
1240:
1237:
1236:
1234:
1232:
1228:
1222:
1215:
1212:
1209:
1207:
1204:
1201:
1198:
1197:
1195:
1193:
1189:
1178:
1175:
1174:
1172:
1170:
1166:
1155:
1152:
1145:
1142:
1135:
1132:
1125:
1122:
1115:
1112:
1111:
1109:
1107:
1103:
1099:
1091:
1086:
1084:
1079:
1077:
1072:
1071:
1068:
1057:
1053:
1049:
1046:
1045:
1044:Chem. Commun.
1040:
1033:
1025:
1021:
1016:
1011:
1006:
1001:
997:
993:
992:
987:
980:
972:
968:
964:
960:
956:
952:
945:
937:
935:9783527296668
931:
927:
923:
919:
912:
904:
900:
895:
890:
886:
882:
878:
874:
870:
863:
855:
851:
847:
843:
842:
834:
826:
822:
818:
814:
806:
799:
793:
789:
788:
783:
776:
768:
764:
760:
756:
755:
747:
739:
735:
731:
727:
723:
720:
719:
711:
703:
699:
695:
689:
685:
684:
676:
668:
666:9789385998898
662:
658:
651:
647:
639:
637:
629:
625:
624:
623:
621:
617:
613:
612:cycloaddition
610:
609:photochemical
606:
602:
597:
595:
590:
588:
584:
580:
576:
572:
571:boronic ester
568:
564:
551:
546:
542:
540:
536:
527:
523:
509:
501:
485:
477:
468:
464:
462:
458:
457:cyclobutanone
454:
444:
431:
429:
425:
410:
406:
405:
404:
402:
401:quadricyclane
398:
397:norbornadiene
394:
384:
380:
379:
375:
371:
370:
363:
359:
358:
355:
351:
348:
347:
331:
330:
326:
323:
321:
318:
316:
312:
309:
306:
302:
299:
298:
297:
289:
286:
284:
280:
279:'large rings'
276:
272:
268:
264:
260:
255:
251:
247:
246:'small rings'
237:
234:
231:
228:
225:
222:
221:
217:
214:
211:
208:
205:
202:
201:
197:
194:
191:
188:
185:
182:
181:
175:
172:
170:
167:
162:
159:
157:
154:
149:
146:
144:
141:
140:
131:
127:
118:
114:
109:
102:
95:
88:
81:
77:
73:
64:
62:
58:
54:
53:concentration
50:
40:
38:
34:
30:
26:
22:
1411:Molecularity
1284:
1047:
1042:
1032:
995:
989:
979:
954:
950:
944:
917:
911:
876:
872:
862:
848:(19): 1167.
845:
839:
833:
819:(19): 3597.
816:
813:J. Org. Chem
812:
805:
786:
782:March, Jerry
775:
758:
752:
746:
721:
716:
710:
682:
675:
656:
650:
633:
598:
591:
582:
579:silyl acetal
560:
549:
532:
473:
450:
421:
390:
295:
287:
282:
278:
270:
266:
262:
258:
254:angle strain
249:
245:
243:
173:
168:
160:
155:
147:
142:
129:
125:
107:
100:
93:
86:
79:
75:
68:
65:
46:
35:of a single
27:describes a
24:
18:
1370:Cage effect
1305:RRKM theory
1221:elimination
575:silyl ether
453:cyclobutane
332:RCHO + CH
232:0.00000001
128:= 5 set to
1529:Categories
718:Chem. Rev.
693:0198503474
642:References
636:exo isomer
616:enantiomer
539:ginkgolide
535:topologies
1421:Catalysis
1317:reactions
998:(3): 21.
601:isopropyl
510:β
486:α
33:structure
21:chemistry
1024:17617903
971:20838677
903:31527923
784:(2007),
738:19873977
702:43338068
399:to give
292:Examples
244:For the
198:0.00001
37:molecule
1039:valinol
1015:1949821
894:6746322
638:forms.
577:, or a
315:indoles
238:0.0003
218:0.0003
135:= 100)
29:process
1096:Basic
1022:
1012:
969:
932:
901:
891:
794:
761:: 55.
736:
700:
690:
663:
581:link (
563:tether
206:0.002
1324:Redox
1160:Acyl)
500:enone
212:0.03
106:>
99:>
92:>
85:>
1213:(E2)
1202:(E1)
1048:1997
1020:PMID
996:2007
967:PMID
930:ISBN
899:PMID
792:ISBN
734:PMID
698:OCLC
688:ISBN
661:ISBN
620:TBAF
455:and
442:edit
372:The
226:100
192:1.7
186:0.1
1183:Ar)
1140:Ar)
1052:doi
1010:PMC
1000:doi
959:doi
922:doi
889:PMC
881:doi
877:110
850:doi
821:doi
763:doi
726:doi
722:110
573:,
541:B.
426:.
313:of
235:15
229:10
215:14
195:12
177:rel
164:rel
151:rel
133:rel
19:In
1531::
1251:(A
1241:(A
1179:(S
1156:(S
1150:i)
1146:(S
1136:(S
1130:2)
1126:(S
1120:1)
1116:(S
1018:.
1008:.
994:.
988:.
965:.
955:39
953:.
928:.
897:.
887:.
875:.
871:.
846:19
844:.
817:47
815:.
759:51
757:.
732:.
696:.
569:,
550:+)
403:.
344:R'
340:CH
223:5
209:7
203:4
189:6
183:3
23:,
1255:)
1253:N
1245:)
1243:E
1219:i
1217:E
1181:E
1158:N
1148:N
1138:N
1128:N
1118:N
1089:e
1082:t
1075:v
1058:.
1054::
1026:.
1002::
973:.
961::
938:.
924::
905:.
883::
856:.
852::
827:.
823::
770:.
765::
740:.
728::
704:.
669:.
446:]
342:2
338:2
334:2
307:.
281:(
269:(
261:(
248:(
174:k
169:n
161:k
156:n
148:k
143:n
130:k
126:n
111:4
108:k
104:7
101:k
97:3
94:k
90:6
87:k
83:5
80:k
76:n
71:n
69:k
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.