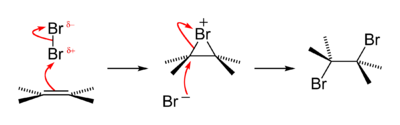134:
124:
330:
279:
256:
193:
on one side of the carbon chain by the first bromine atom and can only attack from the other side. As it attacks and forms a bond with one of the carbons, the bond between the first bromine atom and the other carbon atoms breaks, leaving each carbon atom with a halogen substituent.
111:
for an alkene bromination can be described as follows. In the first step of the reaction, a bromine molecule approaches the electron-rich alkene carbon–carbon double bond. The bromine atom closer to the bond takes on a partial positive charge as its
133:
512:
Olah, George A.; Bollinger, J. Martin (1967). "Stable carbonium ions. XLVIII. Halonium ion formation via neighboring halogen participation. Tetramethylethylene halonium ions".
320:
In an alternative reaction scheme depicted below the reactive intermediate is a β-bromocarbocation or β-bromocarbonium ion with one of the carbon atoms a genuine
181:
When the first bromine atom attacks the carbon–carbon π-bond, it leaves behind one of its electrons with the other bromine that it was bonded to in Br
351:-addition driven by repulsion between the negatively charged carboxylic acid anions being stronger than halonium ion formation. In alkenes such as
485:
Ruasse, Marie
Francoise (1990). "Bromonium ions or β-bromocarbocations in olefin bromination. A kinetic approach to product selectivities".
123:
329:
545:
162:
of the carbon atoms involved. The bonding of bromine is special in this intermediate, due to its relatively large size compared to
174:, making a three-membered ring. The bromide ion acquires a positive formal charge. At this moment the halogen ion is called a "
230:
This reaction mechanism was proposed by
Roberts and Kimball in 1937. With it they explained the observed stereospecific
419:
550:
359:
the substituents are able to stabilize the carbocation by donating electrons at the expense of the halonium ion.
278:
212:
29:
255:
370:
obtained NMR spectra of tetramethylethylenebromonium ions by dissolving 2,3-dibromo-2,3-dimethylbutane in
458:
Fahey, Robert C. (1966). "Polar
Additions to Olefins. II. The Chlorination of Di-t-butylethylene".
154:
at this time and is attacked by the pi electrons of the alkene . It forms for an instant a single
96:
227:, shown to the right below in green), X must attack the bromonium ion from behind, at carbon.
189:
anion and is attracted to the slight positive charge on the carbon atoms. It is blocked from
379:
78:
8:
205:
190:
85:
108:
343:
Roberts and
Kimball in 1937 already accounted for the fact that brominations with the
415:
337:
521:
494:
467:
440:
392:
363:
305:
144:
A bromide ion attacks the C–Br σ* antibonding molecular orbital of a bromonium ion
43:
36:
21:
67:
367:
539:
431:
Roberts, Irving; Kimball, George E. (1937). "The
Halogenation of Ethylenes".
267:
198:
175:
239:
151:
92:
321:
235:
204:
fashion, and when the alkene is part of a cycle the dibromide adopts the
525:
498:
471:
444:
371:
286:
247:
155:
220:
171:
375:
356:
352:
113:
63:
170:
is capable of interacting with both carbons which once shared the
344:
186:
59:
25:
163:
33:
308:
present in the chloronium ion, the only product formed is the
285:
The reaction is even stereospecific in alkenes with two bulky
224:
216:
378:
compound on the other hand was consistent with a rapidly
167:
336:
For reactions taking place through this mechanism no
219:, shown to the right in red) and the nucleophile (X)
116:are repelled by the electrons of the double bond.
246:-double bond forms the dibromide as a mixture of
537:
430:
374:at −60 °C. The spectrum for the corresponding
141:Bromine addition to alkene reaction mechanism
511:
127:Bromine addition to alkene reaction mechanism
362:Halonium ions can be identified by means of
514:Journal of the American Chemical Society
460:Journal of the American Chemical Society
433:Journal of the American Chemical Society
143:
197:In this way the two halogens add in an
66:, and in this case, a solvent could be
538:
484:
457:
315:
211:. For maximum overlap of the C–Br σ*
178:" or "chloronium ion", respectively.
102:
46:of the halogen addition reaction is:
266:-isomer fumaric acid forms a single
185:. That other atom is now a negative
13:
340:is expected and indeed not found.
122:
14:
562:
546:Electrophilic addition reactions
328:
277:
254:
132:
118:
505:
478:
451:
424:
405:
391:Example of bromination in the
382:pair of β-fluorocarbocations.
234:-additions in brominations of
1:
487:Accounts of Chemical Research
398:
213:antibonding molecular orbital
414:4th Ed. Morrison & Boyd
304:-butylethylene. Despite the
296:position as in the compound
7:
385:
91:This type of reaction is a
58:(X represents the halogens
10:
567:
140:
131:
121:
30:carbon–carbon double bond
28:molecule is added to the
18:halogen addition reaction
366:. In 1967 the group of
551:Halogenation reactions
128:
97:electrophilic addition
242:. Maleic acid with a
126:
84:). The product is a
526:10.1021/ja00994a031
499:10.1021/ar00171a006
472:10.1021/ja00972a030
445:10.1021/ja01284a507
191:nucleophilic attack
316:β-Halocarbocations
129:
109:reaction mechanism
103:Reaction mechanism
412:Organic chemistry
338:stereospecificity
148:
147:
558:
530:
529:
509:
503:
502:
482:
476:
475:
455:
449:
448:
428:
422:
409:
393:Auwers synthesis
364:NMR spectroscopy
347:ion resulted in
332:
306:steric repulsion
281:
258:
136:
119:
44:chemical formula
37:functional group
22:organic reaction
566:
565:
561:
560:
559:
557:
556:
555:
536:
535:
534:
533:
510:
506:
483:
479:
456:
452:
429:
425:
410:
406:
401:
388:
318:
184:
105:
82:
75:
71:
53:
12:
11:
5:
564:
554:
553:
548:
532:
531:
504:
477:
450:
423:
403:
402:
400:
397:
396:
395:
387:
384:
368:George A. Olah
334:
333:
317:
314:
283:
282:
260:
259:
182:
166:, the bromide
146:
145:
142:
138:
137:
130:
104:
101:
80:
73:
69:
56:
55:
51:
9:
6:
4:
3:
2:
563:
552:
549:
547:
544:
543:
541:
527:
523:
519:
515:
508:
500:
496:
492:
488:
481:
473:
469:
465:
461:
454:
446:
442:
438:
434:
427:
421:
420:0-205-05838-8
417:
413:
408:
404:
394:
390:
389:
383:
381:
380:equilibrating
377:
373:
369:
365:
360:
358:
354:
350:
346:
341:
339:
331:
327:
326:
325:
323:
313:
311:
307:
303:
299:
295:
291:
289:
280:
276:
275:
274:
272:
270:
265:
257:
253:
252:
251:
249:
245:
241:
237:
233:
228:
226:
222:
218:
214:
210:
209:configuration
208:
203:
201:
195:
192:
188:
179:
177:
176:bromonium ion
173:
169:
165:
161:
157:
153:
152:electrophilic
139:
135:
125:
120:
117:
115:
110:
100:
98:
94:
89:
87:
83:
76:
65:
61:
49:
48:
47:
45:
40:
38:
35:
31:
27:
23:
19:
520:(18): 4744.
517:
513:
507:
490:
486:
480:
466:(20): 4681.
463:
459:
453:
436:
432:
426:
411:
407:
361:
348:
342:
335:
319:
309:
301:
297:
293:
292:groups in a
287:
284:
268:
263:
261:
243:
240:fumaric acid
231:
229:
206:
199:
196:
180:
159:
150:The atom is
149:
106:
93:halogenation
90:
57:
42:The general
41:
20:is a simple
17:
15:
322:carbocation
248:enantiomers
236:maleic acid
540:Categories
439:(5): 947.
399:References
372:magic acid
262:while the
156:sigma bond
88:dihalide.
493:(3): 87.
357:stilbenes
353:anetholes
312:-adduct.
221:lone pair
114:electrons
54:→ X−C−C−X
386:See also
376:fluorine
271:compound
202:addition
64:chlorine
24:where a
345:maleate
187:bromide
95:and an
86:vicinal
60:bromine
50:C=C + X
26:halogen
418:
290:-butyl
172:π-bond
164:carbon
34:alkene
32:of an
264:trans
232:trans
223:(the
215:(the
207:trans
416:ISBN
355:and
310:anti
302:tert
300:-di-
288:tert
269:meso
238:and
225:HOMO
217:LUMO
200:anti
160:both
107:The
522:doi
495:doi
468:doi
441:doi
349:cis
298:cis
294:cis
244:cis
168:ion
158:to
79:CCl
77:or
62:or
542::
518:89
516:.
491:23
489:.
464:88
462:.
437:59
435:.
324:.
273::
250::
99:.
72:Cl
68:CH
39:.
16:A
528:.
524::
501:.
497::
474:.
470::
447:.
443::
183:2
81:4
74:2
70:2
52:2
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.