180:
in the membrane potential. This sudden change in ion concentrations causes the guard cell to shrink which causes the stomata to close which in turn decreases the amount of water lost. All this is a chain reaction according to his research. The increase in ABA causes there to be an increase in calcium ion concentration. Although at first, they thought it was a coincidence they later discovered that this calcium increase is important. They found Ca2+ ions are involved in anion channel activation, which allows for anions to flow into the guard cell. They also are involved in prohibiting proton ATPase from correcting and stopping the membrane from being depolarized. To support their hypothesis that calcium was responsible for all these changes in the cell they did an experiment where they used proteins that inhibited the calcium ions for being produced. If their assumption that calcium is important in these processes they'd see that with the inhibitors they'd see less of the following things. Their assumption was correct and when the inhibitors were used they saw that the proton ATPase worked better to balance the depolarization. They also found that the flow of anions into the guard cells were not as strong. This is important for getting ions to flow into the guard cell. These two things are crucial in causing the stomatal opening to close preventing water loss for the plant.
241:
AtALMT6 is an aluminum-activated malate transporter that is found in guard cells, specifically in the vacuoles. This transport channel was found to cause either an influx or efflux of malate depending on the concentrations of calcium. In a study by Meyer et al, patch-clamp experiments were conducted on mesophyll vacuoles from arabidopsis rdr6-11 (WT) and arabidopsis that were overexpressing AtALMT6-GFP. It was found from these experiments that in the WT there were only small currents when calcium ions were introduced, while in the AtALMT6-GFP mutant a huge inward rectifying current was observed. When the transporter is knocked out from guard cell vacuoles there is a significant reduction in malate flow current. The current goes from a huge inward current to not much different than the WT, and Meyer et al hypothesized that this is due to residual malate concentrations in the vacuole. There is also a similar response in the knockout mutants to drought as in the WT. There was no phenotypic difference observed between the knockout mutants, the wild type, or the AtALMT6-GFP mutants, and the exact cause for this is not fully known.
130:
the protein, which induces H-ATPase activity. The same experiment also found that upon phosphorylation, a 14-3-3 protein was bound to the phototropins before the H-ATPase had been phosphorylated. In a similar experiment they concluded that the binding of 14-3-3 protein to the phosphorylation site is essential for the activation of plasma membrane H-ATPase activity. This was done by adding phosphopeptides such as P-950, which inhibits the binding of 14-3-3 protein, to phosphorylated H-ATPase and observing the amino acid sequence. As protons are being pumped out, a negative electrical potential was formed across the plasma membrane. This hyperpolarization of the membrane allowed the accumulation of charged
189:
31:
1347:
105:. They help to regulate the rate of transpiration by opening and closing the stomata. Light is the main trigger for the opening or closing. Each guard cell has a relatively thick and thinner cuticle on the pore-side and a thin one opposite it. As water enters the cell, the thin side bulges outward like a balloon and draws the thick side along with it, forming a crescent; the combined crescents form the opening of the pore.
237:
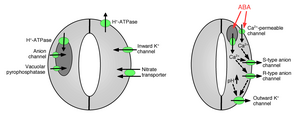
closed. Vascuolar K (VK) channels and fast vacuolar channels can mediate K release from vacuoles. Vacuolar K (VK) channels are activated by elevation in the intracellular calcium concentration. Another type of calcium-activated channel, is the slow vacuolar (SV) channel. SV channels have been shown to function as cation channels that are permeable to Ca ions, but their exact functions are not yet known in plants.
205:, shrinking of the guard cells, and closing of stomatal pores (Figures 1 and 2). Specialized potassium efflux channels participate in mediating release of potassium from guard cells. Anion channels were identified as important controllers of stomatal closing. Anion channels have several major functions in controlling stomatal closing: (a) They allow release of anions, such as chloride and
167:(ABA), is produced in response to drought. A major type of ABA receptor has been identified. The plant hormone ABA causes the stomatal pores to close in response to drought, which reduces plant water loss via transpiration to the atmosphere and allows plants to avoid or slow down water loss during droughts. The use of drought-tolerant
236:
in plants cells. In addition to the ion channels in the plasma membrane, vacuolar ion channels have important functions in regulation of stomatal opening and closure because vacuoles can occupy up to 90% of guard cell's volume. Therefore, a majority of ions are released from vacuoles when stomata are
294:
concentration, which reduces the density of stomatal pores in the surface of leaves in many plant species by presently unknown mechanisms. The genetics of stomatal development can be directly studied by imaging of the leaf epidermis using a microscope. Several major control proteins that function in
268:
intake into plants and plant water loss. Research on guard cell signal transduction mechanisms is producing an understanding of how plants can improve their response to drought stress by reducing plant water loss. Guard cells also provide an excellent model for basic studies on how a cell integrates
179:
ABA is the trigger for the closure of the stomatal opening. To trigger this it activates the release of anions and potassium ions. This influx in anions causes a depolarization of the plasma membrane. This depolarization triggers potassium plus ions in the cell to leave the cell due to the unbalance
145:
pressure of the two guard cells. The turgor pressure of guard cells is controlled by movements of large quantities of ions and sugars into and out of the guard cells. Guard cells have cell walls of varying thickness(its inner region, adjacent to the stomatal pore is thicker and highly cutinized) and
543:
Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, Alfred SE, Bonetta D, Finkelstein R, Provart NJ, Desveaux D, Rodriguez PL, McCourt P, Zhu JK, Schroeder JI, Volkman BF, & Cutler SR (2009) Abscisic acid inhibits type 2C protein phosphatases via
200:
and pumps have been identified and shown to function in the uptake of ions and opening of stomatal apertures. Ion release from guard cells causes stomatal pore closing: Other ion channels have been identified that mediate release of ions from guard cells, which results in osmotic water efflux from
129:
to begin a phosphorylation cascade, which activates H-ATPase, a pump responsible for pumping H ions out of the cell. The phosphorylated H-ATPase allows the binding of a 14-3-3 protein to an autoinhibitory domain of the H-ATPase at the C terminus. Serine and threonine are then phosphorylated within
116:
superfamily. The phototropins trigger many responses such as phototropism, chloroplast movement and leaf expansion as well as stomatal opening. Not much was known about how these photoreceptors worked prior to around 1998. The mechanism by which phototropins work was elucidated through experiments
240:
Guard cells control gas exchange and ion exchange through opening and closing. K+ is one ion that flows both into and out of the cell, causing a positive charge to develop. Malate is one of the main anions used to counteract this positive charge, and it is moved through the AtALMT6 ion channel.
221:. This electrical depolarization of guard cells leads to activation of the outward potassium channels and the release of potassium through these channels. At least two major types of anion channels have been characterized in the plasma membrane: S-type anion channels and R-type anion channels.
253:
concentration, temperature, drought, and plant hormones to trigger cellular responses resulting in stomatal opening or closure. These signal transduction pathways determine for example how quickly a plant will lose water during a drought period. Guard cells have become a model for single cell
714:
Hosy E, Vavasseur A, Mouline K, Dreyer I, Gaymard F, Poree F, Boucherez J, Lebaudy A, Bouchez D, Very AA, Simonneau T, Thibaud JB, & Sentenac H (2003) The
Arabidopsis outward K channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc Natl Acad Sci U S A
171:
plants would lead to a reduction in crop losses during droughts. Since guard cells control water loss of plants, the investigation on how stomatal opening and closure is regulated could lead to the development of plants with improved avoidance or slowing of desiccation and better
666:
Kwak JM, Murata Y, Baizabal-Aguirre VM, Merrill J, Wang M, Kemper A, Hawke SD, Tallman G, & Schroeder JI (2001) Dominant negative guard cell K channel mutants reduce inward-rectifying K currents and light-induced stomatal opening in
Arabidopsis. Plant Physiol.
676:
Lebaudy A, Vavasseur A, Hosy E, Dreyer I, Leonhardt N, Thibaud JB, Very AA, Simonneau T, & Sentenac H (2008) Plant adaptation to fluctuating environment and biomass production are strongly dependent on guard cell potassium channels. Proc. Natl. Acad. Sci. USA
801:
Triin
Vahisalu, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, Lamminmaki A, Brosche M, Moldau H, Desikan R, Schroeder JI, & Kangasjarvi J (2008) SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature
162:
of plants is mediated by several mechanisms that work together, including stabilizing and protecting the plant from damage caused by desiccation and also controlling how much water plants lose through the stomatal pores during drought. A plant hormone,
146:
differently oriented cellulose microfibers, causing them to bend outward when they are turgid, which in turn, causes stomata to open. Stomata close when there is an osmotic loss of water, occurring from the loss of K to neighboring cells, mainly
269:
numerous kinds of input signals to produce a response (stomatal opening or closing). These responses require coordination of numerous cell biological processes in guard cells, including signal reception, ion channel and pump regulation,
138:(Cl) ions, which in turn, increases the solute concentration causing the water potential to decrease. The negative water potential allows for osmosis to occur in the guard cell, so that water enters, allowing the cell to become turgid.
961:
Wang Y, Ying J, Kuzma M, Chalifoux M, Sample A, McArthur C, Uchacz T, Sarvas C, Wan J, Dennis DT, McCourt P, & Huang Y (2005) Molecular tailoring of farnesylation for plant drought tolerance and yield protection. Plant J
289:
During the development of plant leaves, the specialized guard cells differentiate from "guard mother cells". The density of the stomatal pores in leaves is regulated by environmental signals, including increasing atmospheric
704:
Ache P, Becker D, Ivashikina N, Dietrich P, Roelfsema MR, & Hedrich R (2000) GORK, a delayed outward rectifier expressed in guard cells of
Arabidopsis thaliana, is a K-selective, K-sensing ion channel. FEBS Lett
864:
Gobert A, Isayenkov S, Voelker C, Czempinski K, & Maathuis FJ (2007) The two-pore channel TPK1 gene encodes the vacuolar K conductance and plays a role in K homeostasis. Proc Natl Acad Sci U S A 104:10726-10731.
357:
Kwak JM, Mäser P, & Schroeder JI (2008) The clickable guard cell, version II: Interactive model of guard cell signal transduction mechanisms and pathway. The
Arabidopsis Book, eds Last R, Chang C, Graham I,
845:
Ward JM & Schroeder JI (1994) Calcium-activated K channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell
882:
Peiter E, Maathuis FJ, Mills LN, Knight H, Pelloux J, Hetherington AM, & Sanders D (2005) The vacuolar Ca-activated channel TPC1 regulates germination and stomatal movement. Nature 434(7031):404-408.
778:
Pei Z-M, Kuchitsu K, Ward JM, Schwarz M, & Schroeder JI (1997) Differential abscisic acid regulation of guard cell slow anion channels in
Arabdiopsis wild-type and abi1 and abi2 mutants. Plant Cell
439:
Kinoshita, Toshinori; Shimazaki, Ken-ichiro (2002-11-15). "Biochemical
Evidence for the Requirement of 14-3-3 Protein Binding in Activation of the Guard-cell Plasma Membrane H+-ATPase by Blue Light".
811:
Grabov A, Leung J, Giraudat J, & Blatt MR (1997) Alteration of anion channel kinetics in wild-type and abi1-1 transgenic
Nicotiana benthamiana guard cells by abscisic acid. Plant J. 12:203-213.
264:. Cytosolic and nuclear proteins and chemical messengers that function in stomatal movements have been identified that mediate the transduction of environmental signals thus controlling CO
952:
Pei Z-M, Ghassemian M, Kwak CM, McCourt P, & Schroeder JI (1998) Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 282:287-290.
648:
Blatt MR, Thiel G, & Trentham DR (1990) Reversible inactivation of K channels of Vicia stomatal guard cells following the photolysis of caged 1,4,5-trisphosphate. Nature 346:766-769.
112:
proteins which are serine and threonine kinases with blue-light photoreceptor activity. Phototrophins contain two light, oxygen, and voltage sensor (LOV) domains, and are part of the
41:
are specialized plant cells in the epidermis of leaves, stems and other organs that are used to control gas exchange. They are produced in pairs with a gap between them that forms a
534:
Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, & Grill E (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324:1064-1068.
752:
Hedrich R, Busch H, & Raschke K (1990) Ca and nucleotide dependent regulation of voltage dependent anion channels in the plasma membrane of guard cells. EMBO J. 9:3889-3892.
695:
Blatt MR & Armstrong F (1993) K channels of stomatal guard cells: Abscisic-acid-evoked control of the outward-rectifier mediated by cytoplasmic pH. Planta 191:330-341.
820:
Linder B & Raschke K (1992) A slow anion channel in guard cells, activation at large hyperpolarization, may be principal for stomatal closing. FEBS Lett. 131:27-30.
281:
rearrangements and more. A challenge for future research is to assign the functions of some of the identified proteins to these diverse cell biological processes.
516:
Humble GD & Raschke K (1971) Stomatal opening quantitatively related to potassium transport. Evidence from electron probe analysis. Plant
Physiol. 48:447-453.
764:
Schroeder JI & Keller BU (1992) Two types of anion channel currents in guard cells with distinct voltage regulation. Proc. Natl. Acad. Sci. USA 89:5025-5029.
209:
from guard cells, which is needed for stomatal closing. (b) Anion channels are activated by signals that cause stomatal closing, for example by intracellular
618:
Assmann SM, Simoncini L, & Schroeder JI (1985) Blue light activates electrogenic ion pumping in guard cell protoplasts of Vicia faba. Nature 318:285-287.
554:
Meimoun, Patrice; Vidal, Guillaume; Bohrer, Anne-Sophie; Lehner, Arnaud; Tran, Daniel; Briand, Joël; Bouteau, François; Rona, Jean-Pierre (September 2009).
983:
Pillitteri LJ & Torii KU (2007) Breaking the silence: three bHLH proteins direct cell-fate decisions during stomatal development. Bioessays 29:861-870.
213:
and ABA. The resulting release of negatively charged anions from guard cells results in an electrical shift of the membrane to more positive voltages (
657:
Thiel G, MacRobbie EAC, & Blatt MR (1992) Membrane transport in stomatal guard cells: The importance of voltage control. J. Memb. Biol. 126:1-18.
743:
Schroeder JI & Hagiwara S (1989) Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338:427-430.
525:
Schroeder JI, Hedrich R, & Fernandez JM (1984) Potassium-selective single channels in guard cell protoplasts of Vicia faba. Nature 312:361-362.
639:
Schroeder JI, Raschke K, & Neher E (1987) Voltage dependence of K channels in guard cell protoplasts. Proc. Natl. Acad. Sci. USA 84:4108-4112.
319:
Schroeder JI, Kwak JM, & Allen GJ (2001) Guard cell abscisic acid signaling and engineering drought hardiness in plants. Nature 410:327-330.
196:
Ion uptake into guard cells causes stomatal opening: The opening of gas exchange pores requires the uptake of potassium ions into guard cells.
627:
Shimazaki K, Iino M, & Zeiger E (1986) Blue light-dependent proton extrusion by guard-cell protoplasts of Vicia faba. Nature 319:324-326.
89:
absorbed from the air with the water loss through the stomatal pores, and this is achieved by both active and passive control of guard cell
1010:
379:
Kinoshita, Toshinori; Emi, Takashi; Tominaga, Misumi; Sakamoto, Koji; Shigenaga, Ayako; Doi, Michio; Shimazaki, Ken-ichiro (2003-12-01).
81:
of photosynthesis, exits the plant via the stomata. When the stomata are open, water is lost by evaporation and must be replaced via the
727:
Keller BU, Hedrich R, & Raschke K (1989) Voltage-dependent anion channels in the plasma membrane of guard cells. Nature 341:450-453.
686:
Schroeder JI (1988) K transport properties of K channels in the plasma membrane of Vicia faba guard cells. J. Gen. Physiol. 92:667-683.
855:
Allen GJ & Sanders D (1996) Control of ionic currents guard cell vacuoles by cytosolic and luminal calcium. Plant J. 10:1055-1069.
158:
Water stress (drought and salt stress) is one of the major environmental problems causing severe losses in agriculture and in nature.
483:
507:
Imamura S (1943) Untersuchungen uber den mechanismus der turgorschwankung der spaltoffnungs-schliesszellen. Jap. J. Bot. 12:251-346.
903:
Meyer, Stefan; Scholz-Starke, Joachim; Angeli, Alexis De; Kovermann, Peter; Burla, Bo; Gambale, Franco; Martinoia, Enrico (2011).
873:
Hedrich R & Neher E (1987) Cytoplasmic calcium regulates voltage-dependent ion channels in plant vacuoles. Nature 329:833-836.
556:"Intracellular ca2+ stores could participate to abscisic acid-induced depolarization and stomatal closure in Arabidopsis thaliana"
345:
Shimazaki K, Doi M, Assmann SM, & Kinoshita T (2007) Light regulation of stomatal movement. Annu Rev Plant Biol 58:219-247.
381:"Blue-Light- and Phosphorylation-Dependent Binding of a 14-3-3 Protein to Phototropins in Stomatal Guard Cells of Broad Bean"
331:
Hetherington AM & Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424:901-908.
829:
MacRobbie EAC (1998) Signal transduction and ion channels in guard cells. Phil. Trans. Roy. Soc. London 1374:1475-1488.
1003:
788:
Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M, Uchimiya H, Hashimoto M, & Iba K (2008) CO
792:
regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452:483-486.
996:
17:
1350:
905:"Malate transport by the vacuolar AtALMT6 channel in guard cells is subject to multiple regulation"
274:
1027:
904:
1060:
249:
Guard cells perceive and process environmental and endogenous stimuli such as light, humidity, CO
295:
a pathway mediating the development of guard cells and the stomatal pores have been identified.
173:
260:, the investigation of signal processing in single guard cells has become open to the power of
126:
1376:
567:
270:
256:
122:
82:
49:, and closed when water availability is critically low and the guard cells become flaccid.
45:. The stomatal pores are largest when water is freely available and the guard cells become
8:
1200:
571:
1287:
1099:
1094:
1073:
596:
555:
362:, McClung R, & Weinig C (American Society of Plant Biologists, Rockville), pp 1-17.
971:
Bergmann DC & Sack FD (2007) Stomatal development. Annu Rev Plant Biol 58:163-181.
413:
380:
1263:
1035:
935:
927:
923:
601:
583:
464:
456:
418:
400:
197:
159:
1242:
1298:
1019:
919:
591:
575:
448:
408:
392:
66:
1331:
1312:
1269:
1128:
359:
218:
90:
46:
1150:
1174:
1140:
1079:
1050:
214:
58:
50:
30:
1370:
1194:
1084:
1045:
931:
587:
460:
404:
164:
1293:
1236:
1211:
939:
605:
468:
422:
278:
396:
1229:
579:
452:
188:
109:
85:, with water taken up by the roots. Plants must balance the amount of CO
1318:
1274:
1206:
1155:
1145:
1118:
1040:
141:
Opening and closure of the stomatal pore is mediated by changes in the
113:
78:
1355:
1323:
1279:
1216:
988:
233:
147:
131:
54:
1257:
1247:
1169:
1107:
261:
135:
125:
showed blue light excites phototropin 1 and phototropin 2, causing
1113:
902:
229:
210:
202:
1134:
206:
192:
Ion channels and pumps regulating stomatal opening and closure.
142:
70:
739:
737:
735:
733:
1184:
1179:
1163:
102:
42:
544:
the PYR/PYL family of START proteins. Science 324:1068-1071.
378:
1221:
730:
168:
795:
689:
553:
760:
758:
153:
965:
755:
327:
325:
979:
977:
438:
635:
633:
481:
217:) at the intracellular surface of the guard cell
1368:
434:
432:
374:
372:
370:
368:
322:
974:
27:Paired cells that control the stomatal aperture
955:
823:
723:
721:
708:
680:
660:
630:
621:
537:
519:
1004:
946:
898:
896:
894:
892:
890:
888:
429:
365:
867:
841:
839:
837:
835:
746:
670:
612:
65:) from the air through the stomata into the
876:
858:
814:
805:
774:
772:
770:
718:
501:
353:
351:
341:
339:
337:
315:
313:
311:
309:
307:
1011:
997:
885:
849:
651:
642:
510:
832:
782:
698:
595:
412:
224:
183:
767:
348:
334:
304:
187:
29:
528:
101:Guard cells are cells surrounding each
14:
1369:
1018:
244:
96:
992:
154:Water loss and water use efficiency
24:
25:
1388:
1346:
1345:
924:10.1111/j.1365-313X.2011.04587.x
232:are large intracellular storage
547:
484:"Structure of Plants and Fungi"
560:Plant Signaling & Behavior
475:
284:
13:
1:
298:
34:Opening and Closing of Stoma.
7:
10:
1393:
1341:
1059:
1026:
441:Plant and Cell Physiology
93:and stomatal pore size.
482:Digitális Tankönyvtár.
121:). Immunodetection and
225:Vacuolar ion transport
193:
184:Ion uptake and release
35:
397:10.1104/pp.103.029629
191:
127:protein phosphatase 1
33:
580:10.4161/psb.4.9.9396
271:membrane trafficking
257:Arabidopsis thaliana
174:water use efficiency
123:far-western blotting
108:Guard cells contain
83:transpiration stream
1258:Meristematic tissue
572:2009PlSiB...4..830M
488:regi.tankonyvtar.hu
245:Signal transduction
201:guard cells due to
97:Guard cell function
1020:Biological tissues
453:10.1093/pcp/pcf167
198:Potassium channels
194:
36:
1364:
1363:
1151:Phloem parenchyma
912:The Plant Journal
447:(11): 1359–1365.
254:signaling. Using
160:Drought tolerance
117:with broad bean (
77:), produced as a
67:mesophyll tissues
16:(Redirected from
1384:
1349:
1348:
1299:Vascular cambium
1185:Xylem parenchyma
1013:
1006:
999:
990:
989:
984:
981:
972:
969:
963:
959:
953:
950:
944:
943:
909:
900:
883:
880:
874:
871:
865:
862:
856:
853:
847:
843:
830:
827:
821:
818:
812:
809:
803:
799:
793:
786:
780:
776:
765:
762:
753:
750:
744:
741:
728:
725:
716:
712:
706:
702:
696:
693:
687:
684:
678:
674:
668:
664:
658:
655:
649:
646:
640:
637:
628:
625:
619:
616:
610:
609:
599:
551:
545:
541:
535:
532:
526:
523:
517:
514:
508:
505:
499:
498:
496:
495:
479:
473:
472:
436:
427:
426:
416:
391:(4): 1453–1463.
385:Plant Physiology
376:
363:
355:
346:
343:
332:
329:
320:
317:
21:
1392:
1391:
1387:
1386:
1385:
1383:
1382:
1381:
1367:
1366:
1365:
1360:
1337:
1270:Ground meristem
1129:Vascular tissue
1100:Subsidiary cell
1055:
1022:
1017:
987:
982:
975:
970:
966:
960:
956:
951:
947:
907:
901:
886:
881:
877:
872:
868:
863:
859:
854:
850:
844:
833:
828:
824:
819:
815:
810:
806:
800:
796:
791:
787:
783:
777:
768:
763:
756:
751:
747:
742:
731:
726:
719:
713:
709:
703:
699:
694:
690:
685:
681:
675:
671:
665:
661:
656:
652:
647:
643:
638:
631:
626:
622:
617:
613:
552:
548:
542:
538:
533:
529:
524:
520:
515:
511:
506:
502:
493:
491:
480:
476:
437:
430:
377:
366:
356:
349:
344:
335:
330:
323:
318:
305:
301:
293:
287:
267:
252:
247:
227:
219:plasma membrane
186:
156:
99:
91:turgor pressure
88:
76:
64:
53:depends on the
28:
23:
22:
15:
12:
11:
5:
1390:
1380:
1379:
1362:
1361:
1359:
1358:
1353:
1342:
1339:
1338:
1336:
1335:
1328:
1327:
1326:
1321:
1304:
1303:
1302:
1301:
1296:
1284:
1283:
1282:
1277:
1272:
1253:
1252:
1251:
1250:
1245:
1233:
1226:
1225:
1224:
1219:
1214:
1209:
1190:
1189:
1188:
1187:
1182:
1177:
1175:Vessel element
1172:
1160:
1159:
1158:
1153:
1148:
1143:
1141:Companion cell
1124:
1123:
1122:
1121:
1116:
1104:
1103:
1102:
1097:
1092:
1087:
1082:
1080:Bulliform cell
1065:
1063:
1057:
1056:
1054:
1053:
1048:
1043:
1038:
1032:
1030:
1024:
1023:
1016:
1015:
1008:
1001:
993:
986:
985:
973:
964:
954:
945:
918:(2): 247–257.
884:
875:
866:
857:
848:
831:
822:
813:
804:
794:
789:
781:
766:
754:
745:
729:
717:
715:100:5549-5554.
707:
697:
688:
679:
677:105:5271-5276.
669:
659:
650:
641:
629:
620:
611:
566:(9): 830–835.
546:
536:
527:
518:
509:
500:
490:(in Hungarian)
474:
428:
364:
347:
333:
321:
302:
300:
297:
291:
286:
283:
265:
250:
246:
243:
226:
223:
215:depolarization
185:
182:
155:
152:
98:
95:
86:
74:
62:
59:carbon dioxide
51:Photosynthesis
26:
9:
6:
4:
3:
2:
1389:
1378:
1375:
1374:
1372:
1357:
1354:
1352:
1344:
1343:
1340:
1334:
1333:
1329:
1325:
1322:
1320:
1317:
1316:
1315:
1314:
1309:
1306:
1305:
1300:
1297:
1295:
1292:
1291:
1290:
1289:
1285:
1281:
1278:
1276:
1273:
1271:
1268:
1267:
1266:
1265:
1260:
1259:
1255:
1254:
1249:
1246:
1244:
1241:
1240:
1239:
1238:
1234:
1232:
1231:
1227:
1223:
1220:
1218:
1215:
1213:
1210:
1208:
1205:
1204:
1203:
1202:
1197:
1196:
1195:Ground tissue
1192:
1191:
1186:
1183:
1181:
1178:
1176:
1173:
1171:
1168:
1167:
1166:
1165:
1161:
1157:
1154:
1152:
1149:
1147:
1144:
1142:
1139:
1138:
1137:
1136:
1131:
1130:
1126:
1125:
1120:
1117:
1115:
1112:
1111:
1110:
1109:
1105:
1101:
1098:
1096:
1095:Pavement cell
1093:
1091:
1088:
1086:
1083:
1081:
1078:
1077:
1076:
1075:
1070:
1069:Dermal tissue
1067:
1066:
1064:
1062:
1058:
1052:
1049:
1047:
1044:
1042:
1039:
1037:
1034:
1033:
1031:
1029:
1025:
1021:
1014:
1009:
1007:
1002:
1000:
995:
994:
991:
980:
978:
968:
958:
949:
941:
937:
933:
929:
925:
921:
917:
913:
906:
899:
897:
895:
893:
891:
889:
879:
870:
861:
852:
842:
840:
838:
836:
826:
817:
808:
798:
785:
775:
773:
771:
761:
759:
749:
740:
738:
736:
734:
724:
722:
711:
701:
692:
683:
673:
663:
654:
645:
636:
634:
624:
615:
607:
603:
598:
593:
589:
585:
581:
577:
573:
569:
565:
561:
557:
550:
540:
531:
522:
513:
504:
489:
485:
478:
470:
466:
462:
458:
454:
450:
446:
442:
435:
433:
424:
420:
415:
410:
406:
402:
398:
394:
390:
386:
382:
375:
373:
371:
369:
361:
354:
352:
342:
340:
338:
328:
326:
316:
314:
312:
310:
308:
303:
296:
282:
280:
276:
275:transcription
272:
263:
259:
258:
242:
238:
235:
231:
222:
220:
216:
212:
208:
204:
199:
190:
181:
177:
175:
170:
166:
165:abscisic acid
161:
151:
149:
144:
139:
137:
134:(K) ions and
133:
128:
124:
120:
115:
111:
106:
104:
94:
92:
84:
80:
72:
68:
60:
56:
52:
48:
44:
43:stomatal pore
40:
32:
19:
1330:
1311:
1307:
1294:Cork cambium
1286:
1262:
1256:
1237:Sclerenchyma
1235:
1228:
1212:Chlorenchyma
1199:
1193:
1162:
1146:Phloem fiber
1133:
1127:
1106:
1089:
1072:
1068:
967:
957:
948:
915:
911:
878:
869:
860:
851:
825:
816:
807:
802:452:487-491.
797:
784:
748:
710:
700:
691:
682:
672:
667:127:473-485.
662:
653:
644:
623:
614:
563:
559:
549:
539:
530:
521:
512:
503:
492:. Retrieved
487:
477:
444:
440:
388:
384:
288:
279:cytoskeletal
255:
248:
239:
228:
195:
178:
157:
140:
118:
107:
100:
38:
37:
1377:Plant cells
1230:Collenchyma
1180:Xylem fiber
962:43:413-424.
285:Development
110:phototropin
39:Guard cells
18:Guard cells
1319:Endodermis
1275:Procambium
1207:Aerenchyma
1201:Parenchyma
1156:Sieve tube
1119:Phelloderm
1090:Guard cell
1041:Epithelial
1036:Connective
846:6:669-683.
779:9:409-423.
705:486:93-98.
494:2021-04-02
299:References
234:organelles
150:(K) ions.
119:Vicia faba
114:PAS domain
1356:Histology
1324:Exodermis
1288:Secondary
1280:Protoderm
1217:Mesophyll
1074:Epidermis
932:1365-313X
588:1559-2316
461:1471-9053
405:0032-0889
148:potassium
132:potassium
79:byproduct
55:diffusion
1371:Category
1351:Category
1248:Sclereid
1170:Tracheid
1108:Periderm
1046:Muscular
940:21443686
606:19847112
469:12461136
423:14605223
360:Leyser O
262:genetics
230:Vacuoles
136:chloride
1264:Primary
1114:Phellem
1085:Cuticle
1051:Nervous
1028:Animals
597:2802785
568:Bibcode
211:calcium
203:osmosis
1313:Cortex
1135:Phloem
1061:Plants
938:
930:
604:
594:
586:
467:
459:
421:
414:300702
411:
403:
207:malate
143:turgor
71:Oxygen
47:turgid
1332:Stele
1308:Mixed
1243:Fiber
1164:Xylem
908:(PDF)
103:stoma
1222:Pith
936:PMID
928:ISSN
602:PMID
584:ISSN
465:PMID
457:ISSN
419:PMID
401:ISSN
169:crop
920:doi
592:PMC
576:doi
449:doi
409:PMC
393:doi
389:133
61:(CO
57:of
1373::
1310::
1261::
1198::
1132::
1071::
976:^
934:.
926:.
916:67
914:.
910:.
887:^
834:^
769:^
757:^
732:^
720:^
632:^
600:.
590:.
582:.
574:.
562:.
558:.
486:.
463:.
455:.
445:43
443:.
431:^
417:.
407:.
399:.
387:.
383:.
367:^
350:^
336:^
324:^
306:^
290:CO
277:,
273:,
176:.
73:(O
69:.
1012:e
1005:t
998:v
942:.
922::
790:2
608:.
578::
570::
564:4
497:.
471:.
451::
425:.
395::
292:2
266:2
251:2
87:2
75:2
63:2
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.