177:
273:
450:
159:
435:
204:
90:
231:
intermediate when the nucleofuge leaves first or taking place in two steps with an anionic intermediate when the electrofuge leaves first. The carbanionic pathway is more common and is facilitated by the stability of the cation formed and the leaving group ability of the nucleofuge. With cyclic
353:(only one substituent displayed for clarity). The diastereoselectivity of the hydroboration is a result of two factors: avoidance of the axial methyl group as well as axial hydride addition to avoid a twist-boat conformation in the
702:
Ley, S. V.; Antonello, A.; Balskus, E. P.; Booth, D. T.; Christensen, S. B.; Cleator, E.; Gold, H.; Hogenauer, K.; Hunger, U.; Myers, R. M.; Oliver, S. F.; Simic, O.; Smith, M. D.; Sohoel, H.; Woolford, A. J. A. (2004).
446:
has been reported to begin with the reduction of an ether protected amide to form a secondary alcohol. Fragmentation then takes place in a concerted step to form the reaction products.
393:, the mesylate being in the equatorial position allows its sigma star orbital to align ideally with the sigma bond drawn, allowing for the correct olefin geometry seen in
124:
211:
According to reviewers Prantz and Mulzer (2010), the name Grob fragmentation was chosen "in more or less glaring disregard of the earlier contributions".
709:
98:
539:. Comprehensive organic synthesis: Selectivity, strategy, and efficiency in modern organic chemistry. Vol. 6 (1st ed.). Amsterdam:
318:
is a result of borohyride approaching from the bottom face to avoid steric clash with the axial methyl group. Then reduction of the
602:
797:
Wang, Jeh-Jeng; Hu, Wan-Ping (1999). "Novel 3-Aza-Grob
Fragmentation in Hydride Reduction of Ether-Protected Aromatic Lactams".
449:
566:
Strategic applications of named reactions in organic synthesis: Background and detailed mechanisms – 250 named reactions
577:
548:
401:
176:
272:
764:
Wang, Jeh-Jeng; Hu, Wan-Ping; Chung, Hung-Wei; Wang, Li-Fang; Hsu, Mei-Hui (1998). "A new and novel amide bond cleavage of
434:
111:
Alternately, atom 1 could begin as an anion, in which case it becomes neutral rather than going from neutral to cationic.
385:
complex which fragments. As each boron atom can hold three substrate molecules (R), the ultimate boron byproduct is
158:
330:
877:
203:
836:
799:
632:; Frey, A. (1952). "Über die Spaltung des Mesylesters von 2-Methyl-2-oxymethyl-cyclopentanon mit Basen".
262:
89:
596:; Stahly, E. E. (1933). "The Common Basis of Intramolecular Rearrangements. II. The Dehydration of Di-
474:
834:
Hu, Wan-Ping; Wang, Jeh-Jeng; Tsai, Pei-Ching (2000). "Novel
Examples of 3-Aza-Grob Fragmentation".
768:-methoxymethylpyrrolobenzodiazepine-5,11-diones by hydride reduction via 3-aza-Grob fragmentation".
237:
600:-butylcarbinol and the Conversion of the Resulting Nonenes to Trimethylethylene and Isobutylene".
427:
chain with the nitrogen at the 3 position. The reaction products are an electrofugal fragment, an
882:
40:
634:
504:
148:
770:
232:
substrates, the preferred geometry of elimination is for the sigma bond that drives out the
718:
241:
120:
24:
8:
462:
722:
629:
443:
289:
224:
220:
166:
783:
741:
704:
223:
varies with reactant and reaction conditions with the fragmentation taking place in a
853:
816:
746:
684:
593:
573:
544:
281:
258:
140:
132:
845:
808:
779:
736:
726:
676:
667:
643:
611:
513:
479:
386:
362:
354:
103:
338:
665:(2010). "Synthetic Applications of the Carbonyl Generating Grob Fragmentation".
540:
532:
307:
502:
Grob, C. A.; Baumann, W. (1955). "Die 1,4-Eliminierung unter
Fragmentierung".
871:
662:
647:
517:
442:
3-aza-Grob fragmentation can proceed with several different nucleofuges. The
342:
323:
233:
185:
59:
55:
731:
857:
820:
750:
688:
266:
170:
400:
Another example is an epoxy alcohol fragmentation reaction as part of the
416:
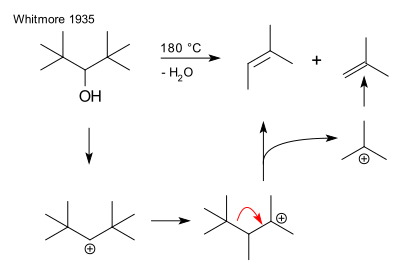
228:
144:
63:
36:
615:
420:
48:
849:
812:
680:
374:
136:
28:
531:
Weyerstahl, P.; Marschall, H. (1991). "Fragmentation
Reactions". In
569:
366:
311:
300:
293:
83:
169:
in 1952 investigated the base catalysed fragmentation of certain
382:
370:
346:
197:
71:
67:
32:
428:
424:
423:
are situated at positions 1 and 5 on a secondary or tertiary
378:
319:
79:
75:
44:
252:
184:
The original work by Grob (1955) concerns the formation of
151:
to a more stable tertiary carbocation and elimination of a
458:
701:
314:. The selectivity of the initial reduction of ketone
143:. This reaction proceeds by formation of a secondary
530:
431:, and a nucleofugal fragment (such as an alcohol).
43:neutral fragment spanning positions 3 and 4, and a
457:The scope of the reaction has been found to cover
869:
465:protecting groups using various hydride agents.
763:
710:Proceedings of the National Academy of Sciences
628:
592:
660:
97:The reaction is named for the Swiss chemist
501:
257:An example of a Grob-like fragmentation in
833:
563:
119:An early instance of fragmentation is the
740:
730:
524:
407:
253:Thapsigargin from Wieland–Miescher ketone
603:Journal of the American Chemical Society
557:
415:is variation which takes place when an
78:; and the negative fragment could be a
54:For example, the positive ion may be a
870:
796:
564:Kürti, László; Czakó, Barbara (2007).
497:
495:
236:to being anti to it, analogous to the
654:
622:
280:In this reaction, diastereoselective
214:
51:") comprising the rest of the chain.
227:or taking place in two steps with a
492:
240:orientation in the E2 mechanism of
66:; the neutral fragment could be an
13:
695:
453:3-Aza-Grob Fragmentation Mechanism
448:
433:
271:
202:
175:
157:
139:, a reaction described in 1933 by
88:
14:
894:
299:, which is functionalized to the
276:Scheme 2. Grob-like fragmentation
705:"Synthesis of the thapsigargins"
827:
438:3-Aza-Grob Fragmentation Scheme
790:
757:
586:
180:Fragmentation Eschenmoser 1952
31:chain into three fragments: a
1:
784:10.1016/S0040-4020(98)00795-9
485:
35:spanning atoms 1 and 2 (the "
837:Journal of Organic Chemistry
800:Journal of Organic Chemistry
402:Holton Taxol total synthesis
357:. The Grob fragmentation to
7:
468:
247:
196:-1,4-dibromocyclohexane by
10:
899:
114:
475:Eschenmoser fragmentation
349:in THF yields the borane
648:10.1002/hlca.19520350532
518:10.1002/hlca.19550380306
413:3-aza-Grob fragmentation
335:-butoxyaluminium hydride
261:is the expansion of the
732:10.1073/pnas.0403300101
537:Heteroatom Manipulation
535:; Fleming, Ian (eds.).
263:Wieland–Miescher ketone
207:Grob fragmentation 1955
635:Helvetica Chimica Acta
543:. pp. 1044–1065.
505:Helvetica Chimica Acta
454:
439:
408:aza-Grob fragmentation
277:
208:
181:
163:
162:Fragmentation Whitmore
149:rearrangement reaction
94:
27:that breaks a neutral
878:Elimination reactions
452:
437:
275:
242:elimination reactions
206:
179:
161:
92:
171:beta hydroxy ketones
25:elimination reaction
778:(43): 13149–13154.
723:2004PNAS..10112073L
717:(33): 12073–12078.
616:10.1021/ja01337a042
463:tetrahydrothiophene
594:Whitmore, Frank C.
455:
444:reaction mechanism
440:
377:group attacks the
290:sodium borohydride
278:
225:concerted reaction
221:reaction mechanism
215:Reaction mechanism
209:
182:
167:Albert Eschenmoser
164:
95:
93:Grob fragmentation
21:Grob fragmentation
850:10.1021/jo000252i
844:(13): 4208–4209.
813:10.1021/jo990549k
807:(15): 5725–5727.
681:10.1021/cr900386h
661:Prantz, Kathrin;
610:(10): 4153–4157.
579:978-0-12-429785-2
550:978-0-08-035929-8
361:takes place with
259:organic synthesis
141:Frank C. Whitmore
133:2-methyl-2-butene
16:Chemical reaction
890:
862:
861:
831:
825:
824:
794:
788:
787:
761:
755:
754:
744:
734:
699:
693:
692:
675:(6): 3741–3766.
668:Chemical Reviews
658:
652:
651:
642:(5): 1660–1666.
626:
620:
619:
590:
584:
583:
561:
555:
554:
528:
522:
521:
499:
480:Wharton reaction
387:trimethyl borate
363:sodium methoxide
355:transition state
107:
898:
897:
893:
892:
891:
889:
888:
887:
868:
867:
866:
865:
832:
828:
795:
791:
762:
758:
700:
696:
659:
655:
630:Eschenmoser, A.
627:
623:
591:
587:
580:
562:
558:
551:
533:Trost, Barry M.
529:
525:
500:
493:
488:
471:
410:
339:tetrahydrofuran
255:
250:
217:
155:-butyl cation:
129:-butyl)methanol
117:
101:
17:
12:
11:
5:
896:
886:
885:
883:Name reactions
880:
864:
863:
826:
789:
756:
694:
663:Mulzer, Johann
653:
621:
585:
578:
556:
549:
541:Pergamon Press
523:
512:(3): 594–610.
490:
489:
487:
484:
483:
482:
477:
470:
467:
409:
406:
381:atom giving a
308:mesyl chloride
284:of the ketone
254:
251:
249:
246:
238:conformational
216:
213:
147:followed by a
116:
113:
15:
9:
6:
4:
3:
2:
895:
884:
881:
879:
876:
875:
873:
859:
855:
851:
847:
843:
839:
838:
830:
822:
818:
814:
810:
806:
802:
801:
793:
785:
781:
777:
773:
772:
767:
760:
752:
748:
743:
738:
733:
728:
724:
720:
716:
712:
711:
706:
698:
690:
686:
682:
678:
674:
670:
669:
664:
657:
649:
645:
641:
638:(in German).
637:
636:
631:
625:
617:
613:
609:
605:
604:
599:
595:
589:
581:
575:
571:
568:. Amsterdam:
567:
560:
552:
546:
542:
538:
534:
527:
519:
515:
511:
508:(in German).
507:
506:
498:
496:
491:
481:
478:
476:
473:
472:
466:
464:
460:
451:
447:
445:
436:
432:
430:
426:
422:
418:
414:
405:
403:
398:
396:
392:
389:. As seen in
388:
384:
380:
376:
372:
368:
364:
360:
356:
352:
348:
344:
343:hydroboration
340:
336:
334:
328:
325:
324:allyl alcohol
321:
317:
313:
309:
305:
302:
298:
295:
291:
287:
283:
274:
270:
268:
264:
260:
245:
243:
239:
235:
234:leaving group
230:
229:carbocationic
226:
222:
212:
205:
201:
199:
195:
191:
187:
186:1,5-hexadiene
178:
174:
172:
168:
160:
156:
154:
150:
146:
142:
138:
134:
130:
128:
122:
112:
109:
105:
100:
99:Cyril A. Grob
91:
87:
85:
81:
77:
73:
69:
65:
61:
57:
52:
50:
46:
42:
38:
34:
30:
26:
22:
841:
835:
829:
804:
798:
792:
775:
769:
765:
759:
714:
708:
697:
672:
666:
656:
639:
633:
624:
607:
601:
597:
588:
565:
559:
536:
526:
509:
503:
456:
441:
412:
411:
399:
394:
390:
358:
350:
341:followed by
332:
326:
315:
303:
296:
285:
279:
267:thapsigargin
256:
218:
210:
193:
189:
183:
165:
152:
126:
118:
110:
96:
53:
45:negative ion
33:positive ion
20:
18:
771:Tetrahedron
417:electrofuge
145:carbocation
121:dehydration
102: [
64:acylium ion
41:unsaturated
37:electrofuge
872:Categories
486:References
421:nucleofuge
49:nucleofuge
375:methoxide
282:reduction
137:isobutene
131:yielding
60:carbonium
56:carbenium
29:aliphatic
858:10866646
821:11674651
751:15226504
689:20163188
570:Elsevier
469:See also
367:methanol
312:pyridine
301:mesylate
248:Examples
84:hydroxyl
719:Bibcode
294:alcohol
292:yields
200:metal:
115:History
39:"), an
856:
819:
749:
742:514437
739:
687:
576:
547:
383:borate
371:reflux
347:borane
198:sodium
72:alkyne
68:alkene
47:(the "
23:is an
429:imine
425:amine
379:boron
345:with
329:with
320:enone
306:with
288:with
194:trans
192:- or
188:from
106:]
86:ion:
80:tosyl
76:imine
74:, or
854:PMID
817:PMID
747:PMID
685:PMID
598:tert
574:ISBN
545:ISBN
461:and
419:and
373:. A
333:tert
331:tri-
219:The
135:and
127:tert
846:doi
809:doi
780:doi
737:PMC
727:doi
715:101
677:doi
673:110
644:doi
612:doi
514:doi
459:THF
397:.
369:at
365:in
337:in
322:to
310:in
265:to
190:cis
125:di(
123:of
82:or
62:or
874::
852:.
842:65
840:.
815:.
805:64
803:.
776:54
774:.
745:.
735:.
725:.
713:.
707:.
683:.
671:.
640:35
608:55
606:.
572:.
510:38
494:^
404:.
269::
244:.
173::
108:.
104:de
70:,
58:,
19:A
860:.
848::
823:.
811::
786:.
782::
766:N
753:.
729::
721::
691:.
679::
650:.
646::
618:.
614::
582:.
553:.
520:.
516::
395:7
391:6
359:6
351:5
327:4
316:1
304:3
297:2
286:1
153:t
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.