502:. Alpha-thalassemia is a genetic blood disorder and one of the most common hemoglobin-related diseases, affecting the production of α subunits from hemoglobin. Depending on how many genes coding for the α subunit are impacted (between one and four), patients with this disease can have reduced to no production of the α subunit of the hemoglobin. As a consequence, less hemoglobin is available and this affects oxygen supply to the tissues. Hemoglobin Barts syndrome manifests when all four genes coding for α subunit are deleted. This is often fatal for the fetus carrying the disorder, as in the absence of α subunits, a form of hemoglobin with four γ subunits, hemoglobin Barts, is produced. This form of hemoglobin isn't fit for oxygen exchange precisely due to its very high affinity for oxygen. While hemoglobin Barts is very efficient at binding oxygen, it doesn't release oxygen to the organs and tissues. The disease is fatal for the fetus or newborn unless early diagnosis and intervention is carried out during pregnancy, and the child will be dependent on lifelong blood transfusions.
606:
other blood-related diseases. In this condition, the genes coding for the γ subunit (HBG1 and HBG2) are not suppressed shortly before birth. This can happen when a mutation occurs in the promoter region of HBG1 and HBG2, preventing the binding of BCL11A and ZBTB7A proteins. These proteins would normally bind and suppress the production of γ subunits and as they can't bind due to the mutation, γ subunits continue to be produced. There are two types of patients with HPFH: either with one normal copy of the gene and one disease form or with two disease copies. Whereas normal adults have less than 1% of hemoglobin F, patients with only one disease gene have 5-30%. Patients with two disease copies can have hemoglobin F in up to 100% of red blood cells. As other diseases such as sickle cell disease could also cause a higher level of hemoglobin F to be present, it can sometimes be misdiagnosed.
450:, which has 3 variants depending on the types of subunits it contains. The production of hemoglobin F starts from week 6, but it's only from 3 months onwards that it becomes the main type found in fetal red blood cells. The switch to produce adult forms of hemoglobin (essentially hemoglobin A) starts at around 40 weeks of gestation, which is close to the expected time of birth. At birth, hemoglobin F accounts for 50-95% of the infant's hemoglobin and at around 6 months after birth, hemoglobin A becomes the predominant type. By the time the baby is one year old, the proportions of different types of hemoglobin are expected to approximate the adult levels, with hemoglobin F reduced to very low levels. The small proportion of red blood cells containing hemoglobin F are called F-cells, which also contain other types of hemoglobin.
554:
hemoglobin F being present and its tendency to be produced only in a subset of cells rather than evenly distributed amongst all red blood cells. In fact, there is a positive correlation between the levels of hemoglobin F and number of F-cells, with patients with higher percentages of hemoglobin F also having a higher proportion of F-cells. Despite the correlations between hemoglobin F levels and F-cell numbers, usually they are determined by direct measurements. While the amount of hemoglobin F is calculated using cell lysates, which are fluids with contents of cells that were broken open, F-cell numbers are done by counting intact red blood cells.
676:, which is a hallmark of the disease. If fetal hemoglobin remains relatively high after birth, the number of painful episodes decreases in patients with sickle-cell disease and they have a better prognosis. Fetal hemoglobin's role in reducing disease severity comes from its ability to disrupt the formation of hemoglobin S chains within red blood cells. Interestingly, while higher levels of hemoglobin F were associated with improvement of some symptoms, including the frequency of painful episodes, leg ulcers and the general severity of the disease, it had no correlation to others. A few examples are
699:
and myeloid leukemia in children, where higher concentrations of hemoglobin F were associated with a worse outcome, including a higher risk of relapse or death. Other cancer types where higher hemoglobin F levels have been observed are transitional cell cancer, colorectal carcinoma and various types of blastomas. In fact, in several types of blastomas, including neuroblastoma and retinoblastoma (affecting the nerve cells and the eyes, respectively), F-cells were found in newly formed blood vessels and spaces in between tumour cells. Clusters of F-cells were also present in the
42:
541:). Secondly, the mother's bloodstream is richer in oxygen than that of the fetus, so oxygen naturally flows towards the fetal circulation by diffusion. The final factor is related to the effects of pH on maternal and fetal hemoglobin. As the maternal blood acquires more carbon dioxide, it becomes more acidic and this favors the release of oxygen by the maternal hemoglobin. At the same time, the decrease in carbon dioxide in fetal blood makes it more alkaline and favors the uptake of oxygen. This is called the Bohr effect or
587:
of F-cells in pregnant women, which was observed between the 23rd to 31st week of gestation. However, as to the reason of the increase in hemoglobin F levels in pregnant women, there doesn't seem to be conclusive evidence. While an early study suggested that maternal red blood cells switch on hemoglobin F production during pregnancy, more recent literature suggested that the increase in haemoglobin F could be, at least in part, due to fetal red blood cells being transferred to the maternal circulation.
469:
428:
591:
it becomes a competition between fetal and maternal hemoglobin F which have similar affinities for oxygen. As a result, women with hemoglobin F as >70% of total hemoglobin are much more likely to have fetuses that are small for their gestational age compared women with <70% hemoglobin F (at a rate of 100% compared to 8%, respectively).
569:. Additionally, some acquired conditions can also have higher F-cell numbers, such as acute erythropoietic stress (response to poor oxygenation which includes very rapid synthesis of new red blood cells) and pregnancy. F-cells have similar mass of haemoglobin per cell compared to red blood cells without haemoglobin F, which is measured
680:, stroke and systemic blood pressure. As hemoglobin F are only produced by some red blood cells, in different quantities, only a subpopulation of cells are protected against sickling. It could be that the symptoms that high hemoglobin F doesn't prevent are quite sensitive to the rupture of the sickled non-F cells.
614:
Delta beta-thalassemia is a rare genetic blood disorder in which the production of both δ and β subunits are reduced or absent. In these cases, the production of the γ subunit increases to compensate for the loss of δ and β subunits, resulting in a higher amount of hemoglobin F present in the blood.
590:
Presence of high levels of hemoglobin F in pregnant women can impact the growth of the fetus, as fetal red blood cells struggle to compete for the oxygen from the mother's circulation. This is because instead of competing with hemoglobin A, which has a weaker association to oxygen than hemoglobin F,
586:
There is a significant increase in hemoglobin F levels during early pregnancy. However, it's not clear whether these levels are stable or decrease as the pregnancy goes on, as different sources reported different results. The increase in hemoglobin F then induces a 3 to 7 fold increase in the number
394:
BCL11A and ZBTB7A are major repressor proteins of hemoglobin F production, by binding to the gene coding for the γ subunit at their promoter region. This happens naturally as the newborn baby starts to switch from producing hemoglobin F to producing hemoglobin A. Some genetic diseases can take place
698:
There have been some studies evaluating the possibility of using hemoglobin F as an indicator of the prognosis for cancer. It has been suggested that elevated concentrations of haemoglobin F can be found in main kinds of solid tumours and blood cancers. Examples include acute lymphoblastic leukemia
646:
The discovery that hemoglobin F alleviated the symptoms of sickle cell disease occurred in 1948. Janet Watson observed that red blood cells from infants with the disease took longer to sickle and did not deform as much compared to their mother's cells, which carried the disease trait. Later, it was
481:
The four hemes, which are the oxygen-binding parts of hemoglobin, are similar between hemoglobin F and other types of hemoglobin, including hemoglobin A. Thus, the key feature that allows hemoglobin F to bind more strongly to oxygen is by having γ subunits (instead of β, for example). In fact, some
605:
This is a rare benign genetic disease where production of hemoglobin F persists after twelve months of life and into the adulthood. As a result, hemoglobin F is present in a higher number of adult red blood cells than normal. It doesn't present symptoms and is usually discovered when screening for
526:
During pregnancy, the mother's circulatory system delivers oxygen and nutrients to the fetus and carries away nutrient-depleted blood enriched with carbon dioxide. The maternal and fetal blood circulations are separate and the exchange of molecules occurs through the placenta, in a region called
703:
of some of these patients. Interestingly, hemoglobin F is not directly produced by tumour cells, but seems to be induced by the biological environment of the cancer in nearby blood cells. A reason suggested for this increase in hemoglobin F is that it may favor cancer growth by providing better
553:
F-cells are the subpopulation of red blood cells that contain hemoglobin F, in amongst other types of hemoglobin. While common in fetuses, in normal adults, only around 3-7% of red blood cells contain hemoglobin F. The low percentage of F-cells in adults owes to two factors: very low levels of
486:
and it enhances hemoglobin's ability to release oxygen. 2,3-BPG interacts much more with hemoglobin A than hemoglobin F. This is because the adult β subunit has more positive charges than the fetal γ subunit, which attract the negative charges from 2,3-BPG. Due to the preference of 2,3-BPG for
510:
To quantify how strongly a certain type of hemoglobin binds to oxygen (or its affinity for oxygen), a parameter called P50 is often used. In a given situation, P50 can be understood as the partial pressure of oxygen at which Hb is 50% saturated. For example, Hemoglobin F has a lower P50 than
511:
hemoglobin A. This means that if we have the same amount of hemoglobin F and hemoglobin A in the blood and add oxygen to it, half of hemoglobin F will bind to oxygen before half of hemoglobin A manages to do so. Therefore, a lower P50 means stronger binding or higher affinity for oxygen.
358:. The protein that they produce is identical, but they differ in gene regulatory regions that determine when or how much of the protein is produced. This leads to HBA1 and HBA2 contributing 40% and 60%, respectively, of the total α subunits produced. As a consequence, mutations on the
534:
Focusing on oxygen exchange, there are three important aspects that allow oxygen to pass from the maternal circulation into the fetal circulation. Firstly, the presence of hemoglobin F in the fetus allows a stronger binding to oxygen than maternal hemoglobin (see
1588:"Fetal hemoglobin-containing cells have the same mean corpuscular hemoglobin as cells without fetal hemoglobin: a reciprocal relationship between gamma- and beta-globin gene expression in normal subjects and in those with high fetal hemoglobin production"
615:
Normally, people have two sets of genes for producing δ and β subunits. People with only one set of working genes don't get any symptoms and in the rarely reported cases where both sets of genes are affected, the patients only experienced mild symptoms.
647:
noted that patients with sickle cell trait as well as hereditary persistence of hemoglobin F (HPFH) didn't have symptoms. Additionally, in sickle cell patients, F-cells were found to be more long living than non-F cells as they contain hemoglobin F.
278:
In the newborn, levels of hemoglobin F gradually decrease and reach adult levels (less than 1% of total hemoglobin) usually within the first year, as adult forms of hemoglobin begin to be produced. Diseases such as
1726:
Boyer SH, Belding TK, Margolte L, Noyes AN, Burke PJ, Bell WR (September 1975). "Variations in the frequency of fetal hemoglobin-bearing erythrocytes (F-cells) in well adults, pregnant women, and adult leukemics".
666:-shaped. These defective red blood cells have a much shorter life span than normal red blood cells (10–20 days compared to up to 120 days). They also have a greater tendency to clump together and block small
704:
oxygen supply to the developing cancerous cells. In adults, increased hemoglobin F production is thought to be caused by factors leading to the activation of the gene coding for the γ subunit, such as
2628:
1670:
Yamada T, Morikawa M, Yamada T, Nishida R, Takeda M, Kawaguchi S, Minakami H (January 2013). "Changes in hemoglobin F levels in pregnant women unaffected by clinical fetomaternal hemorrhage".
1052:
Martyn GE, Wienert B, Yang L, Shah M, Norton LJ, Burdach J, et al. (April 2018). "Natural regulatory mutations elevate the fetal globin gene via disruption of BCL11A or ZBTB7A binding".
2185:
Ma Q, Wyszynski DF, Farrell JJ, Kutlar A, Farrer LA, Baldwin CT, Steinberg MH (December 2007). "Fetal hemoglobin in sickle cell anemia: genetic determinants of response to hydroxyurea".
2325:
Rautonen J, Siimes MA (July 1990). "Initial blood fetal hemoglobin concentration is elevated and is associated with prognosis in children with acute lymphoid or myeloid leukemia".
545:, which also happens in the air exchange in the lungs. All of these three factors are present simultaneously and cooperate to improve the fetus’ access to oxygen from the mother.
629:
267:, allowing it to bind (or attach to) oxygen more strongly; this in turn enables the developing fetus to retrieve oxygen from the mother's bloodstream, which occurs through the
686:
is a chemical that promotes the production of fetal hemoglobin and reduces the premature rupturing of red blood cells. Combination therapy with hydroxyurea and recombinant
1447:
Italia KY, Colah R, Mohanty D (December 2007). "Evaluation of F cells in sickle cell disorders by flow cytometry -- comparison with the
Kleihauer-Betke's slide method".
690:— rather than treatment with hydroxyurea alone — has been shown to further elevate hemoglobin F levels and to promote the development of HbF-containing F-cells.
2230:"Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia"
2584:
1797:
Murji A, Sobel ML, Hasan L, McLeod A, Waye JS, Sermer M, Berger H (February 2012). "Pregnancy outcomes in women with elevated levels of fetal hemoglobin".
2604:
2310:
Wolk M, Newland AC, De La Salle B (1999). "Refinement of plasma fetal hemoglobin (HbF) measurements, as related to whole blood HbF, in cancer patients".
3336:
557:
Due to the correlation between the amount of hemoglobin F and F-cells, F-cell numbers are higher in some inherited hemoglobin disorders, including
514:
For reference, the P50 of fetal hemoglobin is roughly 19 mmHg (a measure of pressure), whereas adult hemoglobin is approximately 26.8 mmHg (see
856:
Sripichai O, Fucharoen S (December 2016). "Fetal hemoglobin regulation in β-thalassemia: heterogeneity, modifiers and therapeutic approaches".
434:
of hemoglobin before and after birth, also showing the cells types and organs where different subunits are being produced over time (data on
3371:
2138:"Fetal hemoglobin in sickle cell anemia: molecular characterization of the unusually high fetal hemoglobin phenotype in African Americans"
2614:
1867:
Thein SL, Craig JE (1998). "Genetics of Hb F/F cell variance in adults and heterocellular hereditary persistence of fetal hemoglobin".
600:
566:
457:
416:
315:
group with an iron element which is key in allowing the binding and unbinding of oxygen. As such, hemoglobin F can adopt two states:
650:
When fetal hemoglobin production is switched off after birth, normal children begin producing adult hemoglobin (HbA). Children with
482:
naturally existing molecules in our body can bind to hemoglobin and change its binding affinity for oxygen. One of the molecules is
2655:
453:
In healthy adults, the composition of hemoglobin is hemoglobin A (~97%), hemoglobin A2 (2.2 - 3.5%) and hemoglobin F (<1%).
1962:
1356:
1331:
1322:
Awasthi V, Goins E, Phillips W (2006). "Chapter 43 – Liposome-encapsulated hemoglobin: history, preparation and evaluation".
1188:
840:
739:
498:
is an abnormal form of hemoglobin produced in hemoglobin Barts syndrome or alpha-thalassemia major, the most severe form of
456:
Certain genetic abnormalities can cause the switch to adult hemoglobin synthesis to fail, resulting in a condition known as
2609:
2629:
Chapter 26 Fetal
Hemoglobin Induction; Management of Sickle-Cell Disease 4th Edition 2002 (NIH Publication No. 02-2117)
2623:
1754:
Dana M, Fibach E (March 2018). "Fetal
Hemoglobin in the Maternal Circulation - Contribution of Fetal Red Blood Cells".
962:
2059:
415:
can cause hemoglobin F to still be produced after the switch to hemoglobin A should have occurred, which is called
331:
subunits, whereas hemoglobin A (97% of total hemoglobin in adults) is composed of two α and two β (beta) subunits.
323:(without oxygen). As hemoglobin F has 4 heme groups, it can bind to up to four oxygen molecules. It is composed of
3356:
1097:"Evaluation of Alpha-Thalassemia Mutations in Cases with Hypochromic Microcytic Anemia: The İstanbul Perspective"
1496:"F-cells in the adult: normal values and levels in individuals with hereditary and acquired elevations of Hb F"
1030:
1347:
Yacov R, Derek K, Namasivayam A (2017). "Chapter 10 – Blood gases: technical aspects and interpretation".
291:, increasing the production of hemoglobin F has been used as a treatment to relieve some of the symptoms.
830:
1205:
2648:
2271:"Augmentation by erythropoietin of the fetal-hemoglobin response to hydroxyurea in sickle cell disease"
1627:
Ibrahim M, Qari MH, Sait W, Abulela M (2009). "Pattern of HB F level rise during normal pregnancies".
729:
1491:
641:
2723:
2801:
2745:
2066:. The Johns Hopkins University, The Johns Hopkins Hospital, and Johns Hopkins Health System. 2020
1604:
1587:
1537:"Stress-associated erythropoiesis initiation is regulated by type 1 conventional dendritic cells"
483:
2782:
1983:
Akinsheye I, Alsultan A, Solovieff N, Ngo D, Baldwin CT, Sebastiani P, et al. (July 2011).
190:
172:
154:
136:
2136:
Akinsheye I, Solovieff N, Ngo D, Malek A, Sebastiani P, Steinberg MH, Chui DH (February 2012).
1512:
1495:
320:
2641:
919:
672:
570:
339:
335:
2633:
3361:
2926:
2912:
2898:
2884:
1206:"Comparing the molecular structure differences between HbF and HbA that affect BPG binding"
447:
446:
During the first 3 months of pregnancy, the main form of hemoglobin in the embryo/fetus is
8:
3192:
3162:
3086:
2728:
2424:"Development of fetal haemoglobin-blood cells (F cells) within colorectal tumour tissues"
2228:
Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, et al. (May 1995).
651:
633:
2476:"Foetal haemoglobin-blood cells (F-cells) as a feature of embryonic tumours (blastomas)"
2034:
1095:
Karakaş Z, Koç B, Temurhan S, Elgün T, Karaman S, Asker G, et al. (December 2015).
902:
Lanzkron S, Strouse JJ, Wilson R, Beach MC, Haywood C, Park H, et al. (June 2008).
255:
weeks of pregnancy and the levels remain high after birth until the baby is roughly 2–4
3176:
2858:
2553:
2526:
2502:
2475:
2448:
2423:
2399:
2372:
2350:
2269:
Rodgers GP, Dover GJ, Uyesaka N, Noguchi CT, Schechter AN, Nienhuis AW (January 1993).
2210:
2162:
2137:
2113:
2086:
2011:
1984:
1930:
1903:
1822:
1779:
1705:
1652:
1563:
1536:
1472:
1299:
1272:
1123:
1096:
1077:
928:
903:
881:
783:
756:
562:
528:
288:
2579:
41:
3157:
3052:
2558:
2507:
2453:
2404:
2342:
2292:
2251:
2202:
2167:
2118:
2087:"Vaso-occlusion in sickle cell disease: pathophysiology and novel targeted therapies"
2016:
1958:
1935:
1884:
1814:
1771:
1736:
1697:
1644:
1609:
1568:
1517:
1464:
1460:
1389:
1372:
Metcalfe J, Bartels H, Moll W (October 1967). "Gas exchange in the pregnant uterus".
1352:
1327:
1304:
1253:
1184:
1128:
1069:
1011:
958:
933:
904:"Systematic review: Hydroxyurea for the treatment of adults with sickle cell disease"
873:
836:
788:
735:
705:
515:
499:
404:
316:
287:, can delay this process, and cause hemoglobin F levels to be higher than normal. In
232:
60:
2354:
2214:
1783:
1709:
1656:
1476:
885:
632:
Increasing the body's production of fetal hemoglobin is used as a strategy to treat
3366:
3062:
2548:
2538:
2497:
2487:
2443:
2435:
2394:
2384:
2334:
2282:
2241:
2194:
2157:
2149:
2108:
2098:
2006:
1996:
1925:
1915:
1876:
1826:
1806:
1763:
1687:
1679:
1636:
1599:
1558:
1548:
1507:
1456:
1420:
1381:
1294:
1284:
1243:
1159:
1118:
1108:
1081:
1061:
1001:
991:
923:
915:
865:
778:
768:
558:
495:
490:
280:
1920:
1767:
1385:
869:
3316:
3171:
2599:
2103:
2001:
1810:
1248:
1231:
1034:
952:
773:
431:
308:
2287:
2270:
2246:
2229:
1289:
3218:
3213:
1425:
1408:
1164:
1147:
996:
979:
687:
659:
542:
366:
gene. There are also two similar copies of the gene coding for the γ subunit,
244:
77:
1953:
Wahed A, Dasgupta A (2015). "Chapter 4 – Hemoglobinopathes and
Thalassemias".
1880:
1683:
1640:
1065:
468:
395:
due to mutations to genes coding for components of hemoglobin F. Mutations to
3350:
3323:
3282:
3197:
3004:
2786:
2769:
2708:
670:, preventing blood supply to tissues and organs. This leads to the so-called
304:
187:
169:
151:
133:
2619:
2439:
1904:"Blessing in disguise; a case of Hereditary Persistence of Fetal Hemoglobin"
3311:
3252:
3128:
3114:
3100:
2990:
2965:
2562:
2511:
2492:
2457:
2408:
2206:
2198:
2171:
2122:
2020:
1939:
1818:
1775:
1701:
1648:
1572:
1468:
1308:
1257:
1132:
1073:
1015:
937:
877:
792:
667:
655:
487:
hemoglobin A, hemoglobin F binds to oxygen with more affinity, in average.
300:
284:
2346:
2296:
2255:
1888:
1613:
1521:
1393:
1113:
3259:
2672:
2389:
1740:
700:
683:
248:
708:(which can activate normally silent genes and is a hallmark of cancer).
427:
3306:
3264:
3167:
2690:
2338:
375:
264:
2543:
2527:"DNA demethylation and invasive cancer: implications for therapeutics"
2153:
1692:
1006:
64:
54:
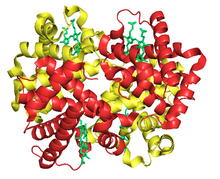
subunits are in red and yellow, respectively, and the iron-containing
3247:
1553:
1841:
1028:
594:
2594:
677:
268:
81:
362:
gene are expected to have a stronger effect than mutations on the
2664:
1672:
Clinica
Chimica Acta; International Journal of Clinical Chemistry
809:
Wang Y, Zhao S (2010). "Chapter 2: Placental Blood
Circulation".
391:
383:
1179:
491:
Even higher oxygen affinity – hemoglobin Barts (four γ subunits)
2681:
663:
272:
229:
91:
628:
531:
which is located in between maternal and fetal blood vessels.
374:, but the protein produced is slightly different, just in one
251:
to organs and tissues in the fetus. It is produced at around 6
2733:
2663:
2373:"Fetal haemopoiesis marking low-grade urinary bladder cancer"
1982:
1489:
1413:
Continuing
Education in Anaesthesia, Critical Care & Pain
1152:
Continuing
Education in Anaesthesia, Critical Care & Pain
236:
2605:
Hemoglobin structure and function (archived
February 3, 2002
1908:
Journal of Community Hospital Internal Medicine Perspectives
2842:
2830:
2825:
2757:
2668:
1669:
757:"Hemoglobin research and the origins of molecular medicine"
354:
348:
343:
312:
247:, and is involved in transporting oxygen from the mother's
182:
164:
146:
128:
102:
55:
2268:
2135:
1535:
Kim TS, Hanak M, Trampont PC, Braciale TJ (October 2015).
901:
3072:
2813:
1725:
2589:
2227:
2184:
1902:
Shaukat I, Pudal A, Yassin S, Höti N, Mustafa S (2018).
1534:
472:
Oxygen-hemoglobin dissociation curves in fetus and adult
1094:
693:
2309:
1901:
1842:"FETAL HEMOGLOBIN QUANTITATIVE TRAIT LOCUS 1; HBFQTL1"
1626:
654:
begin producing a defective form of hemoglobin called
536:
2524:
2027:
1799:
The Journal of Maternal-Fetal & Neonatal Medicine
1796:
1346:
1051:
957:. Hagerstwon, MD: Lippincott Williams & Wilkins.
804:
802:
1321:
576:
476:
2615:
Fetal hemoglobin (doc file; archived March 30 2003)
2610:
Hemoglobin F fact sheet (archived October 29, 2009)
1371:
623:
505:
1446:
855:
799:
263:F has a different composition than adult forms of
2473:
1409:"Placental structure, function and drug transfer"
1406:
595:Hereditary persistence of fetal hemoglobin (HPFH)
3348:
2421:
977:
2084:
1273:"Classification of the disorders of hemoglobin"
2324:
1449:International Journal of Laboratory Hematology
897:
895:
521:
2649:
2525:Cheishvili D, Boureau L, Szyf M (June 2015).
1952:
1442:
1440:
1438:
1436:
1139:
1047:
1045:
1043:
824:
822:
820:
2474:Wolk M, Martin JE, Nowicki M (August 2007).
2318:
1978:
1976:
1974:
1721:
1719:
1605:10.1182/blood.V69.4.1109.bloodjournal6941109
1365:
1229:
748:
723:
721:
68:, by authors Soman, J. and Olson J.S.
2469:
2467:
2366:
2364:
2262:
2221:
1663:
1620:
1277:Cold Spring Harbor Perspectives in Medicine
1264:
1172:
892:
2656:
2642:
2370:
1866:
1753:
1585:
1513:10.1182/blood.V46.5.671.bloodjournal465671
1433:
1270:
1197:
1145:
1088:
1040:
971:
817:
601:Hereditary persistence of fetal hemoglobin
567:hereditary persistence of fetal hemoglobin
458:hereditary persistence of fetal hemoglobin
417:hereditary persistence of fetal hemoglobin
40:
3337:disorders of globin and globulin proteins
2552:
2542:
2518:
2501:
2491:
2447:
2422:Wolk M, Martin JE, Reinus C (June 2006).
2398:
2388:
2286:
2245:
2161:
2112:
2102:
2078:
2010:
2000:
1971:
1929:
1919:
1895:
1860:
1716:
1691:
1603:
1579:
1562:
1552:
1511:
1424:
1400:
1340:
1315:
1298:
1288:
1247:
1223:
1163:
1122:
1112:
1005:
995:
927:
849:
782:
772:
754:
718:
609:
407:and mutations to the promoter regions of
294:
46:Structure of fetal hemoglobin (HbF). The
16:Oxygen carrier protein in the human fetus
2464:
2415:
2361:
2303:
2129:
2085:Manwani D, Frenette PS (December 2013).
1985:"Fetal hemoglobin in sickle cell anemia"
1946:
1790:
1747:
1483:
950:
920:10.7326/0003-4819-148-12-200806170-00221
808:
627:
618:
467:
426:
2590:American Sickle Cell Anemia Association
2052:
1833:
1528:
1178:
442:) Figure last adapted by user Leonid 2.
334:In humans, the α subunit is encoded on
3349:
2178:
1839:
944:
813:. Morgan & Claypool Life Sciences.
658:instead, which form chains that cause
642:Sickle-cell disease § Hydroxyurea
2637:
1541:The Journal of Clinical Investigation
1232:"Gene test review. Alpha-thalassemia"
1203:
984:Blood Cells, Molecules & Diseases
832:Dacie and Lewis Practical Haematology
727:
299:Hemoglobin F, like adult hemoglobin (
1271:Forget BG, Bunn HF (February 2013).
1230:Galanello R, Cao A (February 2011).
978:Farashi S, Harteveld CL (May 2018).
828:
694:Hemoglobin F as a marker for cancers
662:to change their shape from round to
463:
3372:Embryology of cardiovascular system
2275:The New England Journal of Medicine
2234:The New England Journal of Medicine
1349:Assisted Ventilation of the Neonate
581:
382:codes for the protein form with an
311:or chains. Each subunit contains a
13:
2620:Hydroxyurea in sickle-cell disease
1494:, Lim G, Nute PE (November 1975).
980:"Molecular basis of α-thalassemia"
14:
3383:
2573:
2039:U.S. National Library of Medicine
1729:The Johns Hopkins Medical Journal
1586:Dover GJ, Boyer SH (April 1987).
577:Conditions with high hemoglobin F
538:Factors affecting oxygen affinity
484:2,3-bisphosphoglycerate (2,3-BPG)
477:Factors affecting oxygen affinity
283:, which affect components of the
1461:10.1111/j.1365-2257.2006.00884.x
1407:Griffiths S, Campbell J (2015).
811:Vascular Biology of the Placenta
624:Treatment of sickle-cell disease
506:Quantification of oxygen binding
338:and the γ subunit is encoded on
2622:(archived December 28, 2014 at
2531:British Journal of Pharmacology
2371:Wolk M, Martin JE (July 2012).
2142:American Journal of Hematology
1101:Turkish Journal of Haematology
1022:
755:Schechter AN (November 2008).
1:
2595:SCDAA: Break The Sickle Cycle
2585:Transport across the placenta
2428:Journal of Clinical Pathology
1921:10.1080/20009666.2018.1536241
1840:Hemosh A (9 September 2014).
1768:10.1080/03630269.2018.1466712
1386:10.1152/physrev.1967.47.4.782
870:10.1080/17474086.2016.1255142
734:(second ed.). Elsevier.
711:
422:
346:that code for the α subunit,
342:. There are two very similar
110:
2187:The Pharmacogenomics Journal
2104:10.1182/blood-2013-05-498311
2002:10.1182/blood-2011-03-325258
1811:10.3109/14767058.2011.564241
1351:(sixth ed.). Elsevier.
1249:10.1097/GIM.0b013e3181fcb468
774:10.1182/blood-2008-04-078188
7:
2288:10.1056/NEJM199301143280201
2247:10.1056/NEJM199505183322001
1290:10.1101/cshperspect.a011684
1148:"Physiology of haemoglobin"
908:Annals of Internal Medicine
858:Expert Review of Hematology
835:(12th ed.). Elsevier.
522:Oxygen exchange in the womb
10:
3388:
1955:Hematology and Coagulation
1848:. Johns Hopkins University
1146:Thomas C, Lumb AB (2012).
997:10.1016/j.bcmd.2017.09.004
731:Encyclopedia of Immunology
639:
598:
548:
3332:
3299:
3273:
3238:
3231:
3206:
3185:
3150:
3043:
2983:
2944:
2877:
2866:
2857:
2780:
2705:
2698:
2689:
2680:
2480:British Journal of Cancer
2377:British Journal of Cancer
1881:10.3109/03630269809071538
1684:10.1016/j.cca.2012.10.002
1641:10.3109/03630260903332981
1066:10.1038/s41588-018-0085-0
386:at position 136, whereas
109:
98:
87:
73:
39:
26:
21:
1426:10.1093/bjaceaccp/mku013
1165:10.1093/bjaceaccp/mks025
2440:10.1136/jcp.2005.029934
440:Br. Med. Bull. 32, 282.
3357:Protein heteropolymers
2493:10.1038/sj.bjc.6603867
2199:10.1038/sj.tpj.6500433
2064:Johns Hopkins Medicine
637:
610:Delta beta-thalassemia
473:
443:
319:(bound to oxygen) and
295:Structure and genetics
271:found in the mother's
259:months old. Hemoglobin
58:groups in green. From
2060:"Sickle Cell Disease"
2035:"Sickle cell disease"
1374:Physiological Reviews
1114:10.4274/tjh.2014.0204
673:vaso-occlusive crisis
631:
619:Clinical significance
571:mean cell haemoglobin
471:
430:
2600:Hemoglobin synthesis
2390:10.1038/bjc.2012.268
1492:Stamatoyannopoulos G
1236:Genetics in Medicine
951:Costanzo LS (2007).
448:embryonic hemoglobin
243:F is found in fetal
3193:Glycated hemoglobin
3163:Carbaminohemoglobin
652:sickle-cell disease
634:sickle-cell disease
2580:Hemoglobinopathies
2339:10.1007/BF01739428
1326:. Academic press.
1183:. Academic press.
1181:Human Biochemistry
1033:2020-07-31 at the
638:
563:sickle cell anemia
529:intervillous space
474:
444:
289:sickle cell anemia
207:foetal haemoglobin
120:Chromosomal locus
3344:
3343:
3295:
3294:
3291:
3290:
3227:
3226:
3158:Carboxyhemoglobin
3146:
3145:
3039:
3038:
2853:
2852:
2544:10.1111/bph.12885
2154:10.1002/ajh.22221
2041:. NIH. 2020-03-15
1964:978-0-12-800241-4
1358:978-0-323-39006-4
1333:978-0-12-759760-7
1324:Blood Substitutes
1190:978-0-12-383864-3
864:(12): 1129–1137.
842:978-0-7020-6696-2
741:978-0-12-226765-9
706:DNA demethylation
516:Blood gas tension
500:alpha-thalassemia
464:Binding to oxygen
405:alpha-thalassemia
281:beta thalassemias
200:
199:
196:
195:
3379:
3236:
3235:
2875:
2874:
2864:
2863:
2703:
2702:
2696:
2695:
2687:
2686:
2658:
2651:
2644:
2635:
2634:
2567:
2566:
2556:
2546:
2522:
2516:
2515:
2505:
2495:
2471:
2462:
2461:
2451:
2419:
2413:
2412:
2402:
2392:
2368:
2359:
2358:
2322:
2316:
2315:
2312:Journal of Tumor
2307:
2301:
2300:
2290:
2266:
2260:
2259:
2249:
2225:
2219:
2218:
2182:
2176:
2175:
2165:
2133:
2127:
2126:
2116:
2106:
2082:
2076:
2075:
2073:
2071:
2056:
2050:
2049:
2047:
2046:
2031:
2025:
2024:
2014:
2004:
1980:
1969:
1968:
1950:
1944:
1943:
1933:
1923:
1899:
1893:
1892:
1864:
1858:
1857:
1855:
1853:
1837:
1831:
1830:
1794:
1788:
1787:
1751:
1745:
1744:
1723:
1714:
1713:
1695:
1667:
1661:
1660:
1624:
1618:
1617:
1607:
1583:
1577:
1576:
1566:
1556:
1554:10.1172/JCI81919
1532:
1526:
1525:
1515:
1487:
1481:
1480:
1444:
1431:
1430:
1428:
1404:
1398:
1397:
1369:
1363:
1362:
1344:
1338:
1337:
1319:
1313:
1312:
1302:
1292:
1268:
1262:
1261:
1251:
1227:
1221:
1220:
1218:
1216:
1204:Sears D (2016).
1201:
1195:
1194:
1176:
1170:
1169:
1167:
1143:
1137:
1136:
1126:
1116:
1092:
1086:
1085:
1049:
1038:
1026:
1020:
1019:
1009:
999:
975:
969:
968:
948:
942:
941:
931:
899:
890:
889:
853:
847:
846:
826:
815:
814:
806:
797:
796:
786:
776:
752:
746:
745:
728:Linch D (1998).
725:
582:During pregnancy
559:beta-thalassemia
496:Hemoglobin Barts
403:genes can cause
285:adult hemoglobin
262:
258:
254:
242:
203:Fetal hemoglobin
111:
67:
44:
22:Fetal hemoglobin
19:
18:
3387:
3386:
3382:
3381:
3380:
3378:
3377:
3376:
3347:
3346:
3345:
3340:
3328:
3317:Cytochrome P450
3287:
3269:
3223:
3202:
3181:
3172:Deoxyhemoglobin
3142:
3138:
3134:
3124:
3120:
3110:
3106:
3096:
3092:
3082:
3078:
3068:
3058:
3035:
3031:
3027:
3017:
3013:
3008:
3000:
2996:
2979:
2975:
2971:
2961:
2957:
2940:
2936:
2932:
2927:HbE Portland II
2922:
2918:
2908:
2904:
2894:
2890:
2869:
2849:
2776:
2707:Alpha locus on
2676:
2662:
2576:
2571:
2570:
2537:(11): 2705–15.
2523:
2519:
2472:
2465:
2420:
2416:
2369:
2362:
2323:
2319:
2308:
2304:
2267:
2263:
2240:(20): 1317–22.
2226:
2222:
2183:
2179:
2134:
2130:
2083:
2079:
2069:
2067:
2058:
2057:
2053:
2044:
2042:
2033:
2032:
2028:
1981:
1972:
1965:
1951:
1947:
1900:
1896:
1875:(5–6): 401–14.
1865:
1861:
1851:
1849:
1838:
1834:
1795:
1791:
1752:
1748:
1724:
1717:
1668:
1664:
1625:
1621:
1584:
1580:
1547:(10): 3965–80.
1533:
1529:
1488:
1484:
1445:
1434:
1405:
1401:
1370:
1366:
1359:
1345:
1341:
1334:
1320:
1316:
1269:
1265:
1228:
1224:
1214:
1212:
1202:
1198:
1191:
1177:
1173:
1144:
1140:
1093:
1089:
1054:Nature Genetics
1050:
1041:
1035:Wayback Machine
1027:
1023:
976:
972:
965:
949:
945:
900:
893:
854:
850:
843:
829:Wild B (2017).
827:
818:
807:
800:
767:(10): 3927–38.
753:
749:
742:
726:
719:
714:
696:
660:red blood cells
644:
626:
621:
612:
603:
597:
584:
579:
551:
524:
508:
493:
479:
466:
432:Gene expression
425:
321:deoxyhemoglobin
297:
260:
256:
252:
245:red blood cells
240:
233:carrier protein
226:
222:
69:
59:
34:
30:
17:
12:
11:
5:
3385:
3375:
3374:
3369:
3364:
3359:
3342:
3341:
3333:
3330:
3329:
3327:
3326:
3321:
3320:
3319:
3314:
3303:
3301:
3297:
3296:
3293:
3292:
3289:
3288:
3286:
3285:
3279:
3277:
3271:
3270:
3268:
3267:
3262:
3257:
3256:
3255:
3244:
3242:
3233:
3229:
3228:
3225:
3224:
3222:
3221:
3219:Erythrocruorin
3216:
3210:
3208:
3204:
3203:
3201:
3200:
3195:
3189:
3187:
3183:
3182:
3180:
3179:
3177:Sulfhemoglobin
3174:
3165:
3160:
3154:
3152:
3148:
3147:
3144:
3143:
3141:
3140:
3136:
3132:
3126:
3122:
3118:
3112:
3108:
3104:
3098:
3094:
3090:
3084:
3080:
3076:
3070:
3066:
3060:
3056:
3049:
3047:
3041:
3040:
3037:
3036:
3034:
3033:
3029:
3025:
3019:
3015:
3011:
3006:
3002:
2998:
2994:
2987:
2985:
2981:
2980:
2978:
2977:
2973:
2969:
2963:
2959:
2955:
2948:
2946:
2942:
2941:
2939:
2938:
2934:
2930:
2924:
2920:
2916:
2913:HbE Portland I
2910:
2906:
2902:
2896:
2892:
2888:
2881:
2879:
2872:
2861:
2855:
2854:
2851:
2850:
2848:
2847:
2846:
2845:
2835:
2834:
2833:
2828:
2818:
2817:
2816:
2806:
2805:
2804:
2793:
2791:
2778:
2777:
2775:
2774:
2773:
2772:
2762:
2761:
2760:
2750:
2749:
2748:
2738:
2737:
2736:
2731:
2726:
2715:
2713:
2700:
2693:
2684:
2678:
2677:
2661:
2660:
2653:
2646:
2638:
2632:
2631:
2626:
2617:
2612:
2607:
2602:
2597:
2592:
2587:
2582:
2575:
2574:External links
2572:
2569:
2568:
2517:
2463:
2434:(6): 598–602.
2414:
2360:
2317:
2302:
2261:
2220:
2177:
2128:
2097:(24): 3892–8.
2077:
2051:
2026:
1970:
1963:
1945:
1914:(6): 380–381.
1894:
1859:
1832:
1789:
1762:(2): 138–140.
1746:
1715:
1662:
1619:
1598:(4): 1109–13.
1578:
1527:
1482:
1432:
1399:
1380:(4): 782–838.
1364:
1357:
1339:
1332:
1314:
1283:(2): a011684.
1263:
1222:
1196:
1189:
1171:
1158:(5): 251–256.
1138:
1087:
1060:(4): 498–503.
1039:
1021:
970:
964:978-0781773119
963:
943:
914:(12): 939–55.
891:
848:
841:
816:
798:
747:
740:
716:
715:
713:
710:
695:
692:
688:erythropoietin
640:Main article:
625:
622:
620:
617:
611:
608:
599:Main article:
596:
593:
583:
580:
578:
575:
573:values (MCH).
550:
547:
543:Haldane effect
523:
520:
507:
504:
492:
489:
478:
475:
465:
462:
424:
421:
296:
293:
228:) is the main
224:
220:
198:
197:
194:
193:
185:
180:
176:
175:
167:
162:
158:
157:
149:
144:
140:
139:
131:
126:
122:
121:
118:
115:
107:
106:
100:
96:
95:
89:
85:
84:
78:metalloprotein
75:
71:
70:
45:
37:
36:
32:
28:
27:(4 subunits, α
24:
23:
15:
9:
6:
4:
3:
2:
3384:
3373:
3370:
3368:
3365:
3363:
3360:
3358:
3355:
3354:
3352:
3339:
3338:
3331:
3325:
3324:Methemalbumin
3322:
3318:
3315:
3313:
3310:
3309:
3308:
3305:
3304:
3302:
3298:
3284:
3283:Leghemoglobin
3281:
3280:
3278:
3276:
3272:
3266:
3263:
3261:
3258:
3254:
3251:
3250:
3249:
3246:
3245:
3243:
3241:
3237:
3234:
3230:
3220:
3217:
3215:
3214:Chlorocruorin
3212:
3211:
3209:
3205:
3199:
3198:Methemoglobin
3196:
3194:
3191:
3190:
3188:
3184:
3178:
3175:
3173:
3169:
3168:Oxyhemoglobin
3166:
3164:
3161:
3159:
3156:
3155:
3153:
3149:
3130:
3127:
3116:
3113:
3102:
3099:
3088:
3085:
3074:
3071:
3064:
3061:
3054:
3051:
3050:
3048:
3046:
3042:
3023:
3020:
3009:
3003:
2992:
2989:
2988:
2986:
2982:
2967:
2964:
2953:
2950:
2949:
2947:
2943:
2928:
2925:
2914:
2911:
2900:
2897:
2886:
2883:
2882:
2880:
2876:
2873:
2871:
2865:
2862:
2860:
2856:
2844:
2841:
2840:
2839:
2836:
2832:
2829:
2827:
2824:
2823:
2822:
2819:
2815:
2812:
2811:
2810:
2807:
2803:
2800:
2799:
2798:
2795:
2794:
2792:
2790:
2788:
2784:
2779:
2771:
2768:
2767:
2766:
2763:
2759:
2756:
2755:
2754:
2751:
2747:
2744:
2743:
2742:
2739:
2735:
2732:
2730:
2727:
2725:
2722:
2721:
2720:
2717:
2716:
2714:
2712:
2710:
2704:
2701:
2697:
2694:
2692:
2688:
2685:
2683:
2679:
2674:
2670:
2667:that contain
2666:
2659:
2654:
2652:
2647:
2645:
2640:
2639:
2636:
2630:
2627:
2624:
2621:
2618:
2616:
2613:
2611:
2608:
2606:
2603:
2601:
2598:
2596:
2593:
2591:
2588:
2586:
2583:
2581:
2578:
2577:
2564:
2560:
2555:
2550:
2545:
2540:
2536:
2532:
2528:
2521:
2513:
2509:
2504:
2499:
2494:
2489:
2485:
2481:
2477:
2470:
2468:
2459:
2455:
2450:
2445:
2441:
2437:
2433:
2429:
2425:
2418:
2410:
2406:
2401:
2396:
2391:
2386:
2383:(3): 477–81.
2382:
2378:
2374:
2367:
2365:
2356:
2352:
2348:
2344:
2340:
2336:
2332:
2328:
2321:
2313:
2306:
2298:
2294:
2289:
2284:
2280:
2276:
2272:
2265:
2257:
2253:
2248:
2243:
2239:
2235:
2231:
2224:
2216:
2212:
2208:
2204:
2200:
2196:
2193:(6): 386–94.
2192:
2188:
2181:
2173:
2169:
2164:
2159:
2155:
2151:
2147:
2143:
2139:
2132:
2124:
2120:
2115:
2110:
2105:
2100:
2096:
2092:
2088:
2081:
2065:
2061:
2055:
2040:
2036:
2030:
2022:
2018:
2013:
2008:
2003:
1998:
1994:
1990:
1986:
1979:
1977:
1975:
1966:
1960:
1956:
1949:
1941:
1937:
1932:
1927:
1922:
1917:
1913:
1909:
1905:
1898:
1890:
1886:
1882:
1878:
1874:
1870:
1863:
1847:
1843:
1836:
1828:
1824:
1820:
1816:
1812:
1808:
1804:
1800:
1793:
1785:
1781:
1777:
1773:
1769:
1765:
1761:
1757:
1750:
1742:
1738:
1735:(3): 105–15.
1734:
1730:
1722:
1720:
1711:
1707:
1703:
1699:
1694:
1689:
1685:
1681:
1677:
1673:
1666:
1658:
1654:
1650:
1646:
1642:
1638:
1634:
1630:
1623:
1615:
1611:
1606:
1601:
1597:
1593:
1589:
1582:
1574:
1570:
1565:
1560:
1555:
1550:
1546:
1542:
1538:
1531:
1523:
1519:
1514:
1509:
1506:(5): 671–82.
1505:
1501:
1497:
1493:
1486:
1478:
1474:
1470:
1466:
1462:
1458:
1455:(6): 409–14.
1454:
1450:
1443:
1441:
1439:
1437:
1427:
1422:
1418:
1414:
1410:
1403:
1395:
1391:
1387:
1383:
1379:
1375:
1368:
1360:
1354:
1350:
1343:
1335:
1329:
1325:
1318:
1310:
1306:
1301:
1296:
1291:
1286:
1282:
1278:
1274:
1267:
1259:
1255:
1250:
1245:
1241:
1237:
1233:
1226:
1211:
1210:Biosci Portal
1207:
1200:
1192:
1186:
1182:
1175:
1166:
1161:
1157:
1153:
1149:
1142:
1134:
1130:
1125:
1120:
1115:
1110:
1107:(4): 344–50.
1106:
1102:
1098:
1091:
1083:
1079:
1075:
1071:
1067:
1063:
1059:
1055:
1048:
1046:
1044:
1036:
1032:
1029:
1025:
1017:
1013:
1008:
1003:
998:
993:
989:
985:
981:
974:
966:
960:
956:
955:
947:
939:
935:
930:
925:
921:
917:
913:
909:
905:
898:
896:
887:
883:
879:
875:
871:
867:
863:
859:
852:
844:
838:
834:
833:
825:
823:
821:
812:
805:
803:
794:
790:
785:
780:
775:
770:
766:
762:
758:
751:
743:
737:
733:
732:
724:
722:
717:
709:
707:
702:
691:
689:
685:
681:
679:
675:
674:
669:
668:blood vessels
665:
661:
657:
653:
648:
643:
635:
630:
616:
607:
602:
592:
588:
574:
572:
568:
564:
560:
555:
546:
544:
540:
539:
532:
530:
519:
517:
512:
503:
501:
497:
488:
485:
470:
461:
459:
454:
451:
449:
441:
437:
433:
429:
420:
418:
414:
410:
406:
402:
398:
393:
389:
385:
381:
377:
373:
369:
365:
361:
357:
356:
351:
350:
345:
341:
340:chromosome 11
337:
336:chromosome 16
332:
330:
329:two γ (gamma)
327:subunits and
326:
325:two α (alpha)
322:
318:
317:oxyhemoglobin
314:
310:
306:
305:hemoglobin A2
302:
292:
290:
286:
282:
276:
274:
270:
266:
250:
246:
238:
235:in the human
234:
231:
227:
216:
212:
208:
204:
192:
189:
186:
184:
181:
178:
177:
174:
171:
168:
166:
163:
160:
159:
156:
153:
150:
148:
145:
142:
141:
138:
135:
132:
130:
127:
124:
123:
119:
116:
113:
112:
108:
104:
101:
97:
93:
90:
86:
83:
79:
76:
72:
66:
62:
57:
53:
49:
43:
38:
25:
20:
3334:
3312:Cytochrome b
3274:
3253:Metmyoglobin
3239:
3044:
3021:
2951:
2870:development:
2867:
2837:
2820:
2808:
2796:
2781:
2764:
2752:
2740:
2718:
2706:
2673:hemoproteins
2534:
2530:
2520:
2486:(3): 412–9.
2483:
2479:
2431:
2427:
2417:
2380:
2376:
2333:(1): 17–20.
2330:
2326:
2320:
2311:
2305:
2281:(2): 73–80.
2278:
2274:
2264:
2237:
2233:
2223:
2190:
2186:
2180:
2148:(2): 217–9.
2145:
2141:
2131:
2094:
2090:
2080:
2068:. Retrieved
2063:
2054:
2043:. Retrieved
2038:
2029:
1995:(1): 19–27.
1992:
1988:
1957:. Elsevier.
1954:
1948:
1911:
1907:
1897:
1872:
1868:
1862:
1850:. Retrieved
1845:
1835:
1805:(2): 125–9.
1802:
1798:
1792:
1759:
1755:
1749:
1732:
1728:
1675:
1671:
1665:
1635:(6): 534–8.
1632:
1628:
1622:
1595:
1591:
1581:
1544:
1540:
1530:
1503:
1499:
1485:
1452:
1448:
1419:(2): 84–89.
1416:
1412:
1402:
1377:
1373:
1367:
1348:
1342:
1323:
1317:
1280:
1276:
1266:
1239:
1235:
1225:
1213:. Retrieved
1209:
1199:
1180:
1174:
1155:
1151:
1141:
1104:
1100:
1090:
1057:
1053:
1024:
987:
983:
973:
953:
946:
911:
907:
861:
857:
851:
831:
810:
764:
760:
750:
730:
697:
682:
671:
656:hemoglobin S
649:
645:
613:
604:
589:
585:
556:
552:
537:
533:
525:
513:
509:
494:
480:
455:
452:
445:
439:
435:
412:
408:
400:
396:
390:codes for a
387:
379:
376:protein unit
371:
367:
363:
359:
353:
347:
333:
328:
324:
307:), has four
301:hemoglobin A
298:
277:
239:. Hemoglobin
218:
214:
211:hemoglobin F
210:
206:
202:
201:
114:Subunit name
74:Protein type
51:
47:
3362:Hemoglobins
3260:Neuroglobin
3186:Other human
2899:HbE Gower 2
2885:HbE Gower 1
1242:(2): 83–8.
701:bone marrow
684:Hydroxyurea
249:bloodstream
99:Cofactor(s)
3351:Categories
3307:Cytochrome
3265:Cytoglobin
3045:pathology:
2868:stages of
2783:Beta locus
2691:Hemoglobin
2045:2020-03-15
1869:Hemoglobin
1756:Hemoglobin
1693:2115/53256
1629:Hemoglobin
1007:1887/79403
954:Physiology
712:References
438:, (1976).
423:Production
265:hemoglobin
94:-transport
3335:see also
3248:Myoglobin
3151:Compounds
3022:HbF/Fetal
2952:HbF/Fetal
2878:Embryonic
2859:Tetramers
1678:: 124–7.
1490:Wood WG,
990:: 43–53.
436:Wood W.G.
3207:Nonhuman
2699:Subunits
2665:Proteins
2563:25134627
2512:17595660
2458:16469830
2409:22735903
2355:22096967
2215:33180368
2207:17299377
2172:22139998
2123:24052549
2070:16 April
2021:21490337
1940:30559951
1852:15 March
1819:21473677
1784:13661613
1776:29745271
1710:23746089
1702:23073220
1657:41124341
1649:19958203
1573:26389678
1477:46171087
1469:17988294
1309:23378597
1258:21381239
1215:11 March
1133:26377141
1074:29610478
1031:Archived
1016:29032940
938:18458272
886:10820279
878:27801605
793:18988877
678:priapism
309:subunits
269:placenta
88:Function
82:globulin
3367:Infancy
2682:Globins
2554:4439869
2503:2360326
2449:1860403
2400:3405209
2347:1696840
2297:7677965
2256:7715639
2163:3302931
2114:3854110
2012:3139383
1931:6292363
1889:9859924
1827:5500015
1614:2435342
1564:4607133
1522:1100141
1394:4964061
1300:3552344
1124:4805326
1082:4690503
929:3256736
784:2581994
549:F-cells
392:glycine
384:alanine
188:Chr. 11
170:Chr. 11
152:Chr. 16
134:Chr. 16
3275:plant:
3240:human:
2734:pseudo
2561:
2551:
2510:
2500:
2456:
2446:
2407:
2397:
2353:
2345:
2295:
2254:
2213:
2205:
2170:
2160:
2121:
2111:
2019:
2009:
1961:
1938:
1928:
1887:
1825:
1817:
1782:
1774:
1741:810611
1739:
1708:
1700:
1655:
1647:
1612:
1571:
1561:
1520:
1475:
1467:
1392:
1355:
1330:
1307:
1297:
1256:
1187:
1131:
1121:
1080:
1072:
1014:
961:
936:
926:
884:
876:
839:
791:
781:
738:
664:sickle
273:uterus
261:
257:
253:
241:
230:oxygen
209:(also
92:oxygen
3300:Other
3232:Other
3063:Barts
2984:Adult
2945:Fetal
2351:S2CID
2211:S2CID
2091:Blood
1989:Blood
1823:S2CID
1780:S2CID
1706:S2CID
1653:S2CID
1592:Blood
1500:Blood
1473:S2CID
1078:S2CID
882:S2CID
761:Blood
344:genes
237:fetus
217:, or
205:, or
191:p15.4
179:Hb-γ2
173:p15.4
161:Hb-γ1
155:p13.3
143:Hb-α2
137:p13.3
125:Hb-α1
2843:HBE1
2831:HBG2
2826:HBG1
2758:HBQ1
2729:HBA2
2724:HBA1
2669:heme
2559:PMID
2508:PMID
2454:PMID
2405:PMID
2343:PMID
2327:Blut
2293:PMID
2252:PMID
2203:PMID
2168:PMID
2119:PMID
2072:2020
2017:PMID
1959:ISBN
1936:PMID
1885:PMID
1854:2020
1846:OMIM
1815:PMID
1772:PMID
1737:PMID
1698:PMID
1645:PMID
1610:PMID
1569:PMID
1518:PMID
1465:PMID
1390:PMID
1353:ISBN
1328:ISBN
1305:PMID
1254:PMID
1217:2020
1185:ISBN
1129:PMID
1070:PMID
1012:PMID
959:ISBN
934:PMID
874:PMID
837:ISBN
789:PMID
736:ISBN
565:and
413:HBG2
411:and
409:HBG1
401:HBA2
399:and
397:HBA1
388:HBG2
380:HBG1
372:HBG2
370:and
368:HBG1
364:HBA1
360:HBA2
355:HBA2
352:and
349:HBA1
313:heme
303:and
183:HBG2
165:HBG1
147:HBA2
129:HBA1
117:Gene
103:heme
65:4MQJ
56:heme
50:and
3129:HbO
3115:HbE
3101:HbC
3087:HbS
3073:HbD
3053:HbH
3005:HbA
2991:HbA
2966:HbA
2814:HBD
2802:HBB
2785:on
2770:HBM
2746:HBZ
2549:PMC
2539:doi
2535:172
2498:PMC
2488:doi
2444:PMC
2436:doi
2395:PMC
2385:doi
2381:107
2335:doi
2283:doi
2279:328
2242:doi
2238:332
2195:doi
2158:PMC
2150:doi
2109:PMC
2099:doi
2095:122
2007:PMC
1997:doi
1993:118
1926:PMC
1916:doi
1877:doi
1807:doi
1764:doi
1733:137
1688:hdl
1680:doi
1676:415
1637:doi
1600:doi
1559:PMC
1549:doi
1545:125
1508:doi
1457:doi
1421:doi
1382:doi
1295:PMC
1285:doi
1244:doi
1160:doi
1119:PMC
1109:doi
1062:doi
1002:hdl
992:doi
924:PMC
916:doi
912:148
866:doi
779:PMC
769:doi
765:112
518:).
215:HbF
105:(4)
61:PDB
3353::
3131:(α
3117:(α
3103:(α
3089:(α
3075:(α
3065:(γ
3055:(β
3024:(α
3010:(α
2993:(α
2968:(α
2954:(α
2929:(ζ
2915:(ζ
2901:(α
2887:(ζ
2787:11
2709:16
2557:.
2547:.
2533:.
2529:.
2506:.
2496:.
2484:97
2482:.
2478:.
2466:^
2452:.
2442:.
2432:59
2430:.
2426:.
2403:.
2393:.
2379:.
2375:.
2363:^
2349:.
2341:.
2331:61
2329:.
2291:.
2277:.
2273:.
2250:.
2236:.
2232:.
2209:.
2201:.
2189:.
2166:.
2156:.
2146:87
2144:.
2140:.
2117:.
2107:.
2093:.
2089:.
2062:.
2037:.
2015:.
2005:.
1991:.
1987:.
1973:^
1934:.
1924:.
1910:.
1906:.
1883:.
1873:22
1871:.
1844:.
1821:.
1813:.
1803:25
1801:.
1778:.
1770:.
1760:42
1758:.
1731:.
1718:^
1704:.
1696:.
1686:.
1674:.
1651:.
1643:.
1633:33
1631:.
1608:.
1596:69
1594:.
1590:.
1567:.
1557:.
1543:.
1539:.
1516:.
1504:46
1502:.
1498:.
1471:.
1463:.
1453:29
1451:.
1435:^
1417:15
1415:.
1411:.
1388:.
1378:47
1376:.
1303:.
1293:.
1279:.
1275:.
1252:.
1240:13
1238:.
1234:.
1208:.
1156:12
1154:.
1150:.
1127:.
1117:.
1105:32
1103:.
1099:.
1076:.
1068:.
1058:50
1056:.
1042:^
1010:.
1000:.
988:70
986:.
982:.
932:.
922:.
910:.
906:.
894:^
880:.
872:.
860:.
819:^
801:^
787:.
777:.
763:.
759:.
720:^
561:,
460:.
419:.
378::
275:.
213:,
80:,
63::
52:2γ
48:2α
3170:/
3139:)
3137:2
3135:β
3133:2
3125:)
3123:2
3121:β
3119:2
3111:)
3109:2
3107:β
3105:2
3097:)
3095:2
3093:β
3091:2
3083:)
3081:2
3079:β
3077:2
3069:)
3067:4
3059:)
3057:4
3032:)
3030:2
3028:γ
3026:2
3018:)
3016:2
3014:δ
3012:2
3007:2
3001:)
2999:2
2997:β
2995:2
2976:)
2974:2
2972:β
2970:2
2962:)
2960:2
2958:γ
2956:2
2937:)
2935:2
2933:β
2931:2
2923:)
2921:2
2919:γ
2917:2
2909:)
2907:2
2905:ε
2903:2
2895:)
2893:2
2891:ε
2889:2
2838:ε
2821:γ
2809:δ
2797:β
2789::
2765:μ
2753:θ
2741:ζ
2719:α
2711::
2675:)
2671:(
2657:e
2650:t
2643:v
2625:)
2565:.
2541::
2514:.
2490::
2460:.
2438::
2411:.
2387::
2357:.
2337::
2314:.
2299:.
2285::
2258:.
2244::
2217:.
2197::
2191:7
2174:.
2152::
2125:.
2101::
2074:.
2048:.
2023:.
1999::
1967:.
1942:.
1918::
1912:8
1891:.
1879::
1856:.
1829:.
1809::
1786:.
1766::
1743:.
1712:.
1690::
1682::
1659:.
1639::
1616:.
1602::
1575:.
1551::
1524:.
1510::
1479:.
1459::
1429:.
1423::
1396:.
1384::
1361:.
1336:.
1311:.
1287::
1281:3
1260:.
1246::
1219:.
1193:.
1168:.
1162::
1135:.
1111::
1084:.
1064::
1037:.
1018:.
1004::
994::
967:.
940:.
918::
888:.
868::
862:9
845:.
795:.
771::
744:.
636:.
225:2
223:γ
221:2
219:α
35:)
33:2
31:γ
29:2
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.