126:
452:
253:
30:
185:
352:
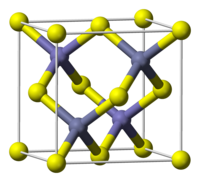
motif, which is not observed for dihalides, the metals exhibit trigonal prismatic structures. The strong bonding between the metal and chalcogenide ligands, contrasts with the weak chalcogenide—chalcogenide bonding between the layers. Owing to these contrasting bond strengths, these materials engage
140:
Transition metal chalcogenides occur with many stoichiometries and many structures. Most common and most important technologically, however, are the chalcogenides of simple stoichiometries, such as 1:1 and 1:2. Extreme cases include metal-rich phases (e.g.
200:
In most of their chalcogenides, transition metals adopt oxidation states of II or greater. Nonetheless, several examples exist where the metallic atoms far outnumber the chalcogens. Such compounds typically have extensive metal-metal bonding.
361:. The intercalation process is accompanied by charge transfer, reducing the M(IV) centers to M(III). The attraction between electrons and holes in 2D tungsten diselenide is 100s of times stronger than in a typical 3D semiconductor.
377:. The sulfur atoms within the persulfido dianion are bound together via a short S-S bond. "Late" transition metal disulfides (Mn, Fe, Co, Ni) almost always adopt the pyrite or the related
209:
Metal monochalcogenides have the formula ME, where M = a transition metal and E = S, Se, Te. They typically crystallize in one of two motifs, named after the corresponding forms of
108:
Alkali metal and alkaline earth monochalcogenides are salt-like, being colourless and often water-soluble. The sulfides tend to undergo hydrolysis to form derivatives containing
268:, where M = a transition metal and E = S, Se, Te. The most important members are the sulfides. They are always dark diamagnetic solids, insoluble in all solvents, and exhibit
279:
In terms of their electronic structures, these compounds are usually viewed as derivatives of M, where M = Ti (d configuration), V (d configuration), Mo (d configuration).
225:
structure wherein the atom connectivities are similar (tetrahedral), but the crystal symmetry is hexagonal. A third motif for metal monochalcogenide is the
229:
lattice, where the metal and chalcogenide each have octahedral and trigonal prismatic coordination, respectively. This motif is commonly subject to
799:
809:
Kovalenko, Maksym V.; Scheele, Marcus; Talapin, Dmitri V. (2009). "Colloidal
Nanocrystals with Molecular Metal Chalcogenide Surface Ligands".
433:, is an example of a metal tetrachalcogenide. Crystallographic analysis shows that the material can be considered a bis(persulfide), i.e. V,(S
389:
Several metals, mainly for the early metals (Ti, V, Cr, Mn groups) also form trichalcogenides. These materials are usually described as M(E
217:
structure, the sulfide atoms pack in a cubic symmetry and the Zn ions occupy half of the tetrahedral holes. The result is a
532:. The structures of many main group materials are dictated by directional covalent bonding, rather than by close packing.
763:
746:
730:
624:
600:
595:
Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the
Elements (2nd Edn.), Oxford:Butterworth-Heinemann.
381:
motif, in contrast to early metals (V, Ti, Mo, W) which adopt 4+ oxidation state with two chalcogenide dianions.
699:
Franzen, Hugo F. (1978). "Structure and
Bonding of Metal-Rich Compounds: Pnictides, chalcogenides and halides".
619:
Vaughan, D. J.; Craig, J. R. "Mineral
Chemistry of Metal Sulfides" Cambridge University Press, Cambridge: 1978.
17:
853:
295:. Molybdenum disulfide is the subject of thousands of articles and the main ore of molybdenum, termed
354:
288:
156:
For the purpose of classifying these materials, the chalcogenide is often viewed as a dianion, i.e.,
58:
of the periodic table are defined as chalcogens, the term chalcogenide is more commonly reserved for
511:
637:
Hughbanks, Timothy (1995). "Exploring the metal-rich chemistry of the early transition elements".
481:
except the noble gases. Usually, their stoichiometries follow the classical valence trends, e.g.
869:
725:"Sulfide Mineralogy: Volume 1" Paul H. Ribbe, editor, 1974, Mineralogical Society of America.
559:
544:
315:
165:
67:
818:
535:
The chalcogen is assigned positive oxidation states for the halides, nitrides, and oxides.
341:
304:
145:
S), which exhibit extensive metal-metal bonding, and chalcogenide-rich materials such as Re
90:
8:
569:
564:
500:
394:
322:
308:
822:
89:. Some pigments and catalysts are also based on chalcogenides. The metal dichalcogenide
842:
554:
478:
402:
373:, a common mineral, is usually described as consisting of Fe and the persulfido anion S
280:
82:
834:
742:
726:
712:
650:
620:
596:
482:
273:
79:
846:
826:
708:
681:
646:
337:
230:
55:
800:
Phase change memory-based 'moneta' system points to the future of computer storage
300:
241:
226:
117:
97:
51:
125:
33:
Cadmium sulfide, a prototypical metal chalcogenide, is used as a yellow pigment.
664:
Franzen, H.F.; Beineke, T.A.; Conrad, B.R. (1968). "The crystal structure of Nb
685:
863:
489:
358:
269:
830:
741:
Wells, A.F. (1984) Structural
Inorganic Chemistry, Oxford: Clarendon Press.
838:
221:
framework. The main alternative structure for the monochalcogenides is the
210:
112:(SH) anions. The alkali metal chalcogenides often crystallize with the anti
426:
370:
296:
214:
451:
129:
The zinc blende structure is a common motif for metal monochalcogenides.
468:
307:. The corresponding diselenides and even ditellurides are known, e.g.,
252:
218:
86:
663:
287:
for secondary batteries, exploiting its ability to reversibly undergo
549:
378:
109:
44:
260:, the most common metal dichalcogenide, adopts a layered structure.
222:
173:
169:
161:
113:
71:
63:
29:
464:
292:
284:
237:
157:
59:
103:
176:, not ionic, as indicated by their semiconducting properties.
75:
47:
522:
348:
motif, the metals exhibit octahedral structures. In the MoS
184:
336:
Transition metal dichalcogenides typically adopt either
153:, which features extensive chalcogen-chalcogen bonding.
808:
791:
790:
172:. In fact, transition metal chalcogenides are highly
393:)(E) (where E = S, Se, Te). A well known example is
43:is a chemical compound consisting of at least one
135:
861:
698:
369:In contrast to classical metal dichalcogenides,
364:
477:Chalcogen derivatives are known for all of the
384:
116:structure and the alkaline earth salts in the
854:Big Blue boffins hatch dirt-cheap solar cells
104:Alkali metal and alkaline earth chalcogenides
764:"Physics Duo Finds Magic in Two Dimensions"
591:
589:
587:
585:
444:
244:. Many minerals and ores are monosulfides.
179:
78:. Many metal ores exist as chalcogenides.
636:
264:Metal dichalcogenides have the formula ME
236:Important monochalcogenides include some
582:
450:
251:
183:
124:
28:
615:
613:
611:
609:
14:
862:
510:. Many exceptions exist however, e.g.
188:Structure of the metal-rich sulfide Nb
761:
757:
755:
606:
467:where the As and S centers obey the
331:
204:
24:
247:
25:
881:
784:
752:
701:Progress in Solid State Chemistry
639:Journal of Alloys and Compounds
856:The Register, 12 February 2010
735:
719:
692:
657:
630:
283:was investigated in prototype
136:Transition metal chalcogenides
13:
1:
575:
365:Pyrite and related disulfides
762:Wood, Charlie (2022-08-16).
713:10.1016/0079-6786(78)90002-X
651:10.1016/0925-8388(95)01688-0
401:is produced by treatment of
7:
538:
385:Tri- and tetrachalcogenides
10:
886:
429:, which has the formula VS
686:10.1107/S0567740868002463
674:Acta Crystallographica B
445:Main group chalcogenides
180:Metal-rich chalcogenides
831:10.1126/science.1170524
472:
344:structures. In the CdI
261:
197:
130:
54:element. Although all
50:and at least one more
34:
560:Hydrogen chalcogenide
545:Carbon dichalcogenide
454:
272:properties. Some are
255:
187:
128:
32:
342:molybdenum disulfide
305:hydrodesulfurization
83:chalcogenide glasses
823:2009Sci...324.1417K
817:(5933): 1417–1420.
570:Phase-change memory
565:Negative resistance
479:main group elements
395:niobium triselenide
555:Chalcogenide glass
473:
403:tetrathiomolybdate
299:. It is used as a
281:Titanium disulfide
262:
198:
131:
35:
463:is a crosslinked
332:Transition metals
303:and catalyst for
205:Monochalcogenides
56:group 16 elements
16:(Redirected from
877:
850:
778:
777:
775:
774:
759:
750:
739:
733:
723:
717:
716:
696:
690:
689:
661:
655:
654:
634:
628:
617:
604:
593:
338:cadmium diiodide
231:nonstoichiometry
21:
885:
884:
880:
879:
878:
876:
875:
874:
860:
859:
787:
782:
781:
772:
770:
768:Quanta Magazine
760:
753:
740:
736:
724:
720:
697:
693:
680:(3): 412–p416.
671:
667:
662:
658:
635:
631:
618:
607:
594:
583:
578:
541:
530:
526:
519:
515:
508:
504:
497:
493:
486:
462:
458:
447:
440:
436:
432:
420:
416:
412:
400:
397:. Amorphous MoS
392:
387:
376:
367:
351:
347:
334:
326:
319:
312:
301:solid lubricant
274:superconductors
267:
259:
250:
248:Dichalcogenides
242:cadmium sulfide
227:nickel arsenide
207:
195:
191:
182:
152:
148:
144:
138:
118:sodium chloride
106:
98:solid lubricant
94:
80:Photoconductive
52:electropositive
23:
22:
15:
12:
11:
5:
883:
873:
872:
858:
857:
851:
806:
797:
786:
785:External links
783:
780:
779:
751:
734:
718:
691:
669:
665:
656:
629:
605:
580:
579:
577:
574:
573:
572:
567:
562:
557:
552:
547:
540:
537:
528:
524:
517:
513:
506:
502:
495:
491:
484:
475:
474:
460:
456:
446:
443:
438:
434:
430:
423:
422:
418:
414:
410:
398:
390:
386:
383:
374:
366:
363:
349:
345:
333:
330:
324:
317:
310:
270:semiconducting
265:
257:
249:
246:
206:
203:
193:
189:
181:
178:
150:
146:
142:
137:
134:
133:
132:
105:
102:
92:
74:, rather than
37:
36:
9:
6:
4:
3:
2:
882:
871:
870:Chalcogenides
868:
867:
865:
855:
852:
848:
844:
840:
836:
832:
828:
824:
820:
816:
812:
807:
804:
801:
798:
795:
792:
789:
788:
769:
765:
758:
756:
748:
747:0-19-855370-6
744:
738:
732:
731:0-939950-01-4
728:
722:
714:
710:
706:
702:
695:
687:
683:
679:
675:
660:
652:
648:
644:
640:
633:
626:
625:0-521-21489-0
622:
616:
614:
612:
610:
602:
601:0-7506-3365-4
598:
592:
590:
588:
586:
581:
571:
568:
566:
563:
561:
558:
556:
553:
551:
548:
546:
543:
542:
536:
533:
531:
520:
509:
498:
487:
480:
470:
466:
453:
449:
448:
442:
428:
408:
407:
406:
404:
396:
382:
380:
372:
362:
360:
359:alkali metals
356:
355:intercalation
343:
339:
329:
327:
320:
313:
306:
302:
298:
294:
290:
289:intercalation
286:
282:
277:
275:
271:
254:
245:
243:
239:
234:
232:
228:
224:
220:
216:
212:
202:
186:
177:
175:
171:
167:
163:
159:
154:
127:
123:
122:
121:
119:
115:
111:
101:
99:
95:
88:
84:
81:
77:
73:
69:
65:
61:
57:
53:
49:
46:
42:
31:
27:
26:
19:
18:Chalcogenides
814:
810:
805:Jun 03, 2011
802:
796:Jun 14, 2016
793:
771:. Retrieved
767:
737:
721:
704:
700:
694:
677:
673:
659:
642:
638:
632:
534:
476:
425:The mineral
424:
388:
368:
335:
278:
263:
235:
211:zinc sulfide
208:
199:
155:
139:
107:
96:is a common
85:are used in
41:chalcogenide
40:
38:
803:ScienceBlog
413:+ 2 H → MoS
405:with acid:
371:iron pyrite
297:molybdenite
215:zinc blende
773:2022-08-22
576:References
469:octet rule
240:, notably
219:diamondoid
87:xerography
68:tellurides
645:: 40–53.
550:Chalcogen
427:patrónite
379:marcasite
213:. In the
110:bisulfide
72:polonides
64:selenides
45:chalcogen
864:Category
847:21845356
839:19520953
707:: 1–39.
539:See also
285:cathodes
238:pigments
223:wurtzite
174:covalent
114:fluorite
60:sulfides
819:Bibcode
811:Science
794:ACTAlab
465:polymer
293:lithium
120:motif.
845:
837:
745:
729:
623:
599:
321:, and
168:, and
76:oxides
70:, and
843:S2CID
48:anion
835:PMID
743:ISBN
727:ISBN
621:ISBN
597:ISBN
521:and
316:MoSe
309:TiSe
827:doi
815:324
709:doi
682:doi
672:".
647:doi
643:229
483:SiS
417:+ H
409:MoS
357:by
353:in
340:or
323:WSe
291:by
256:MoS
91:MoS
866::
841:.
833:.
825:.
813:.
766:.
754:^
705:12
703:.
678:24
676:.
666:21
641:.
608:^
584:^
501:Sb
499:,
488:,
455:As
441:.
328:.
314:,
276:.
233:.
190:21
170:Po
166:Te
164:,
162:Se
160:,
141:Ta
100:.
66:,
62:,
39:A
849:.
829::
821::
776:.
749:.
715:.
711::
688:.
684::
670:8
668:S
653:.
649::
627:.
603:.
529:4
527:N
525:4
523:S
518:3
516:S
514:4
512:P
507:3
505:S
503:2
496:3
494:S
492:2
490:B
485:2
471:.
461:3
459:S
457:2
439:2
437:)
435:2
431:4
421:S
419:2
415:3
411:4
399:3
391:2
375:2
350:2
346:2
325:2
318:2
311:2
266:2
258:2
196:.
194:8
192:S
158:S
151:7
149:S
147:2
143:2
93:2
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.