712:
therapy. This approval amends the indication previously granted under accelerated approval for people with metastatic urothelial carcinoma with susceptible FGFR3 or FGFR2 alterations after prior platinum-containing chemotherapy. Efficacy was evaluated in Study BLC3001 Cohort 1, a randomized, open-label trial of 266 participants with metastatic urothelial carcinoma harboring selected FGFR3 alterations who had received 1-2 prior systemic treatments, including a PD-1 or PD-L1 inhibitor. Participants were randomized 1:1 to receive erdafitinib or investigator's choice of chemotherapy (docetaxel or vinflunine). Randomization was stratified by region, performance status, and presence of visceral or bone metastases. FGFR3 alterations were identified from tumor tissue in a central laboratory by the therascreen FGFR RGQ RT-PCR kit (Qiagen) in 75% of participants, while the remainder were identified by local next generation sequencing assays.
470:
447:
42:
2844:
1243:
1170:
1017:
689:
Erdafitinib may cause serious eye problems, including inflamed eyes, inflamed cornea (front part of the eye) and disorders of the retina, an internal part of the eye. Patients are advised to have eye examinations intermittently and to tell their health care professional right away if they develop
685:
Common side effects include increased phosphate level, mouth sores, feeling tired, change in kidney function, diarrhea, dry mouth, nails separating from the bed or poor formation of the nail, change in liver function, low salt (sodium) levels, decreased appetite, change in sense of taste, low red
744:
adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product
Balversa, intended for the treatment of urothelial carcinoma harbouring susceptible FGFR3 genetic alterations. The applicant for this medicinal product is Janssen-Cilag International N.V.
698:
The efficacy of erdafitinib was studied in a clinical trial (NCT02365597) that included 87 adults with locally advanced or metastatic bladder cancer, with FGFR3 or FGFR2 genetic alterations, that had progressed following treatment with chemotherapy. The overall response rate in these adults was
711:
In
January 2024, the FDA approved erdafitinib for adults with locally advanced or metastatic urothelial carcinoma with susceptible FGFR3 genetic alterations, as determined by an FDA-approved companion diagnostic test, whose disease has progressed on or after at least one line of prior systemic
673:
In
January 2024, the FDA approved erdafitinib for adults with locally advanced or metastatic urothelial carcinoma with susceptible FGFR3 genetic alterations, as determined by an FDA-approved companion diagnostic test, whose disease has progressed on or after at least one line of prior systemic
1061:
707:
designation. The FDA granted the approval of
Balversa to Janssen Pharmaceutical. The FDA also approved the therascreen FGFR RGQ RT-PCR Kit, developed by Qiagen Manchester, Ltd., for use as a companion diagnostic with erdafinitib for this therapeutic indication.
949:
1053:
699:
32.2%, with 2.3% having a complete response and almost 30% having a partial response. The response lasted for a median of approximately five-and-a-half months. The trial was conducted in Asia, Europe, and the United States.
702:
Erdafitinib received an accelerated approval. Further clinical trials are required to confirm erdafitinib's clinical benefit and the sponsor is conducting or plans to conduct these studies. Erdafitinib was also granted
686:
blood cells (anemia), dry skin, dry eyes and hair loss. Other side effects include redness, swelling, peeling or tenderness on the hands or feet (hand foot syndrome), constipation, stomach pain, nausea and muscle pain.
650:
for the treatment of adults with locally advanced or metastatic urothelial carcinoma with susceptible FGFR3 genetic alterations whose disease has progressed on or after at least one line of prior systemic therapy.
1358:
for "A Study of
Erdafitinib Compared With Vinflunine or Docetaxel or Pembrolizumab in Participants With Advanced Urothelial Cancer and Selected Fibroblast Growth Factor Receptor (FGFR) Gene Aberrations (THOR)" at
941:
1002:
1268:
539:
1054:"Balversa (erdafitinib) Receives U.S. FDA Approval for the Treatment of Patients with Locally Advanced or Metastatic Urothelial Carcinoma with Certain FGFR Genetic Alterations"
1108:
1151:
1423:
1395:
for "A Study to
Evaluate the Safety, Pharmacokinetics, and Pharmacodynamics of JNJ-42756493 in Adult Participants With Advanced or Refractory Solid Tumors or Lymphoma" at
1292:"Multicenter Phase I Study of Erdafitinib (JNJ-42756493), Oral Pan-Fibroblast Growth Factor Receptor Inhibitor, in Patients with Advanced or Refractory Solid Tumors"
677:
In August 2024, the EMA approved erdafitinib for adults with unresectable or metastatic urothelial carcinoma (mUC) carrying susceptible FGFR3 genetic alterations.
158:
581:
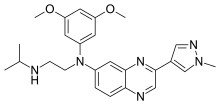
InChI=1S/C25H30N6O2/c1-17(2)26-8-9-31(20-10-21(32-4)13-22(11-20)33-5)19-6-7-23-24(12-19)29-25(15-27-23)18-14-28-30(3)16-18/h6-7,10-17,26H,8-9H2,1-5H3
990:
2874:
1416:
1260:
1187:
737:
897:
Text was copied from this source which is copyright
European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
1857:
1842:
662:
with an FGFR3 or FGFR2 alteration that has progressed beyond traditional platinum-based therapies, subject to a confirmatory trial. The U.S.
72:
553:
1409:
813:
1228:
2415:
2058:
844:
942:"Janssen Announces U.S. FDA Breakthrough Therapy Designation for Erdafitinib in the Treatment of Metastatic Urothelial Cancer"
1551:
2869:
2864:
2529:
123:
608:
573:
1949:
1475:
1086:
241:
188:
104:
786:
2295:
1371:
for "An
Efficacy and Safety Study of Erdafitinib (JNJ-42756493) in Participants With Urothelial Cancer" at
1222:
1114:
1092:
996:
663:
655:
465:
366:
910:
415:
2476:
1452:
426:
1401:
2834:
2601:
2450:
2199:
1701:
762:
1383:
for "A Study of
Erdafitinib in Participants With Metastatic or Locally Advanced Urothelial Cancer" at
1926:
1792:
1505:
882:
741:
667:
442:
315:
1936:
1462:
1261:"Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 24-27 June 2024"
17:
1432:
631:
134:
54:
2304:
1787:
1516:
604:
355:
2884:
1862:
1802:
1772:
1675:
1511:
877:
624:
267:
1717:
1612:
1449:
1216:
726:
704:
616:
375:
469:
446:
8:
2570:
1837:
1827:
1647:
1290:
Bahleda R, Italiano A, Hierro C, Mita A, Cervantes A, Chan N, et al. (August 2019).
258:
141:
2879:
2225:
1762:
1396:
1384:
1372:
1360:
1331:
1188:"Erdafitinib Wins EU Approval for FGFR3+ Unresectable, Metastatic Urothelial Carcinoma"
754:
1035:
1902:
1335:
1323:
766:
730:
292:
214:
202:
96:
1742:
1584:
1579:
1313:
1303:
635:
612:
482:
171:
82:
1391:
1379:
1367:
1354:
1308:
1291:
753:
Researchers have investigated erdafitinib for safety and efficacy in treatment of
2848:
2679:
2577:
2525:
2123:
1436:
758:
745:
Erdafitinib was approved for medical use in the
European Union in August 2024.
659:
627:
2858:
2736:
2626:
2588:
2565:
2497:
2492:
2482:
2258:
2254:
2242:
2049:
1832:
1797:
1617:
1597:
1247:
1174:
1021:
818:
791:
458:
33:
2776:
2761:
2731:
2711:
2593:
2541:
2502:
2390:
2369:
2276:
2238:
2139:
2114:
2110:
1994:
1959:
1912:
1897:
1852:
1727:
1697:
1622:
1440:
1327:
620:
166:
2806:
2786:
2781:
2771:
2751:
2721:
2704:
2652:
2642:
2637:
2632:
2534:
2461:
2431:
2426:
2421:
2405:
2395:
2375:
2250:
2246:
2234:
2230:
2159:
2063:
2009:
2004:
1969:
1907:
1892:
1882:
1877:
1872:
1867:
1847:
1812:
1767:
1747:
1737:
1692:
1657:
1637:
1593:
1556:
1544:
1539:
1501:
1485:
1431:
90:
335:
2821:
2816:
2791:
2756:
2741:
2716:
2699:
2689:
2647:
2618:
2583:
2549:
2466:
2456:
2436:
2400:
2385:
2380:
2350:
2284:
2280:
2262:
2210:
2189:
2149:
2144:
2083:
2014:
1989:
1964:
1887:
1822:
1782:
1777:
1757:
1752:
1732:
1722:
1712:
1627:
1607:
1589:
1497:
515:
346:
148:
1318:
2811:
2801:
2796:
2766:
2746:
2694:
2684:
2672:
2667:
2613:
2608:
2554:
2487:
2360:
2355:
2340:
2335:
2330:
2320:
2315:
2310:
2215:
2205:
2184:
2179:
2174:
2169:
2164:
2154:
2134:
2098:
2093:
2088:
2078:
2073:
2040:
2035:
2030:
1999:
1979:
1974:
1817:
1807:
1632:
1480:
647:
278:
76:
2561:
2325:
2129:
2068:
2024:
1984:
1954:
1707:
1571:
1567:
1492:
991:"FDA approves first targeted therapy for metastatic bladder cancer"
395:
326:
118:
1081:
1079:
538:
1665:
1246:
This article incorporates text from this source, which is in the
1173:
This article incorporates text from this source, which is in the
1020:
This article incorporates text from this source, which is in the
1076:
611:(FGFR) used for the treatment of cancer. FGFRs are a subset of
561:
CC(C)NCCN(C1=CC2=NC(=CN=C2C=C1)C3=CN(N=C3)C)C4=CC(=CC(=C4)OC)OC
406:
41:
2545:
2271:
2105:
2054:
1534:
529:
431:
249:
N'-(3,5-dimethoxyphenyl)-N'--N-propan-2-ylethane-1,2-diamine
2660:
2446:
1944:
1670:
1652:
1642:
1602:
1470:
386:
654:
In April 2019, erdafitinib was granted approval by the US
1289:
615:
which are unregulated in some tumors and influence tumor
690:
blurred vision, loss of vision or other visual changes.
658:(FDA) for treatment of metastatic or locally advanced
2832:
839:
837:
1152:"FDA approves erdafitinib for urothelial carcinoma"
834:
2856:
314:
291:
1211:
1209:
1146:
1144:
1142:
1140:
1138:
1136:
1134:
1087:"Drug Approval Package: Balversa (erdafinitib)"
872:
870:
868:
866:
266:
814:"Summary Basis of Decision (SBD) for Balversa"
738:Committee for Medicinal Products for Human Use
1417:
905:
903:
1206:
1131:
863:
122:
845:"Balversa- erdafitinib tablet, film coated"
1424:
1410:
900:
468:
445:
354:
40:
1317:
1307:
1036:"Pyrazolyl quinazoline kinase inhibitors"
985:
983:
981:
979:
936:
934:
932:
374:
2875:Drugs developed by Johnson & Johnson
977:
975:
973:
971:
969:
967:
965:
963:
961:
959:
729:designation by the FDA for treatment of
725:In March 2018, erdafitinib was granted
441:
334:
95:
14:
2857:
1231:from the original on 27 September 2019
929:
715:
646:In the United Statest, erdafitinib is
607:. It is a small molecule inhibitor of
459:
220:
1405:
1033:
1005:from the original on 15 November 2019
956:
414:
208:
113:
81:
915:Union Register of medicinal products
305:
170:
394:
282:
24:
952:from the original on 20 June 2018.
25:
2896:
1346:
1271:from the original on 12 July 2024
1217:"Drug Trials Snapshots: Balversa"
1156:U.S. Food and Drug Administration
1109:"New Drug Therapy Approvals 2019"
609:fibroblast growth factor receptor
2842:
1241:
1168:
1015:
1001:(Press release). 12 April 2019.
499:
493:
1283:
1253:
1180:
1101:
1064:from the original on 8 May 2019
720:
680:
641:
586:Key:OLAHOMJCDNXHFI-UHFFFAOYSA-N
1046:
1027:
911:"Balversa Product information"
806:
787:"Balversa Product information"
779:
505:
487:
197:
13:
1:
1309:10.1158/1078-0432.CCR-18-3334
1060:(Press release). 8 May 2019.
772:
1223:Food and Drug Administration
1115:Food and Drug Administration
1093:Food and Drug Administration
997:Food and Drug Administration
664:Food and Drug Administration
656:Food and Drug Administration
630:the drug and licensed it to
599:, sold under the brand name
7:
1034:Saxty G (3 November 2011).
748:
666:(FDA) considers it to be a
10:
2901:
1927:Tyrosine kinase inhibitors
763:non-small cell lung cancer
693:
477:Chemical and physical data
2870:Protein kinase inhibitors
2865:Experimental cancer drugs
2517:
2294:
1935:
1925:
1793:Mirvetuximab soravtansine
1685:
1566:
1526:
1461:
1448:
1265:European Medicines Agency
883:European Medicines Agency
742:European Medicines Agency
668:first-in-class medication
569:
549:
527:
514:
481:
476:
457:
425:
405:
385:
365:
345:
325:
302:
277:
257:
237:
232:
187:
182:
157:
147:
133:
103:
89:
71:
63:
53:
48:
39:
1937:Receptor tyrosine kinase
1463:Receptor tyrosine kinase
153:Organonitrogen compounds
1527:Others for solid tumors
1433:Targeted cancer therapy
632:Janssen Pharmaceuticals
1788:Loncastuximab tesirine
1517:Trastuzumab deruxtecan
1389:Clinical trial number
1377:Clinical trial number
1365:Clinical trial number
1352:Clinical trial number
605:anti-cancer medication
1863:Sacituzumab govitecan
1803:Moxetumomab pasudotox
1773:Inotuzumab ozogamicin
1676:Gemtuzumab ozogamicin
1512:Trastuzumab emtansine
1453:monoclonal antibodies
1437:antineoplastic agents
1058:Johnson & Johnson
946:Johnson & Johnson
625:Astex Pharmaceuticals
623:, and cell survival.
1718:Belantamab mafodotin
727:breakthrough therapy
705:breakthrough therapy
617:cell differentiation
2571:Denileukin diftitox
2233:(ALK, ROS1, NTRK),
1838:Polatuzumab vedotin
1828:Oportuzumab monatox
716:Society and culture
36:
2257:(ROS1, TRK, ALK),
1763:Enfortumab vedotin
1397:ClinicalTrials.gov
1385:ClinicalTrials.gov
1373:ClinicalTrials.gov
1361:ClinicalTrials.gov
1119:. 31 December 2019
736:In June 2024, the
32:
2830:
2829:
2513:
2512:
1921:
1920:
1903:Tisotumab vedotin
1227:. 12 April 2019.
1158:. 19 January 2024
948:(Press release).
851:. 1 February 2024
822:. 23 October 2014
767:esophageal cancer
731:urothelial cancer
619:, proliferation,
594:
593:
540:Interactive image
427:CompTox Dashboard
224:
212:
200:
116:
27:Chemical compound
16:(Redirected from
2892:
2847:
2846:
2845:
2838:
2544:peptide against
2117:(AXL, ALK, LTK))
1933:
1932:
1743:Dinutuximab beta
1459:
1458:
1426:
1419:
1412:
1403:
1402:
1340:
1339:
1321:
1311:
1296:Clin. Cancer Res
1287:
1281:
1280:
1278:
1276:
1267:. 28 June 2024.
1257:
1251:
1245:
1244:
1240:
1238:
1236:
1213:
1204:
1203:
1201:
1199:
1194:. 23 August 2024
1184:
1178:
1172:
1171:
1167:
1165:
1163:
1148:
1129:
1128:
1126:
1124:
1105:
1099:
1098:
1083:
1074:
1073:
1071:
1069:
1050:
1044:
1043:
1031:
1025:
1019:
1018:
1014:
1012:
1010:
987:
954:
953:
938:
927:
926:
924:
922:
917:. 23 August 2024
907:
898:
896:
894:
892:
874:
861:
860:
858:
856:
841:
832:
831:
829:
827:
810:
804:
803:
801:
799:
783:
755:bile duct cancer
613:tyrosine kinases
542:
522:
507:
501:
495:
489:
472:
461:
450:
449:
435:
433:
418:
398:
378:
358:
338:
318:
308:
307:
295:
285:
284:
270:
222:
219:
210:
207:
199:
196:
174:
126:
115:
112:
99:
85:
44:
37:
35:
31:
21:
2900:
2899:
2895:
2894:
2893:
2891:
2890:
2889:
2855:
2854:
2853:
2843:
2841:
2833:
2831:
2826:
2680:Pi3K inhibitors
2578:mTOR inhibitors
2509:
2290:
2261:(VEGFR, FGFR),
1917:
1681:
1562:
1522:
1444:
1430:
1349:
1344:
1343:
1302:(16): 4888–97.
1288:
1284:
1274:
1272:
1259:
1258:
1254:
1242:
1234:
1232:
1215:
1214:
1207:
1197:
1195:
1186:
1185:
1181:
1169:
1161:
1159:
1150:
1149:
1132:
1122:
1120:
1107:
1106:
1102:
1085:
1084:
1077:
1067:
1065:
1052:
1051:
1047:
1032:
1028:
1016:
1008:
1006:
989:
988:
957:
940:
939:
930:
920:
918:
909:
908:
901:
890:
888:
878:"Balversa EPAR"
876:
875:
864:
854:
852:
843:
842:
835:
825:
823:
812:
811:
807:
797:
795:
785:
784:
780:
775:
751:
723:
718:
696:
683:
644:
590:
587:
582:
577:
576:
565:
562:
557:
556:
545:
520:
510:
504:
498:
492:
453:
443:DTXSID001027936
429:
421:
401:
381:
361:
341:
321:
304:
298:
281:
273:
253:
250:
245:
244:
228:
178:
136:
129:
28:
23:
22:
15:
12:
11:
5:
2898:
2888:
2887:
2882:
2877:
2872:
2867:
2852:
2851:
2828:
2827:
2825:
2824:
2819:
2814:
2809:
2804:
2799:
2794:
2789:
2784:
2779:
2774:
2769:
2764:
2759:
2754:
2749:
2744:
2739:
2734:
2729:
2724:
2719:
2714:
2709:
2708:
2707:
2702:
2697:
2692:
2687:
2677:
2676:
2675:
2670:
2657:
2656:
2655:
2650:
2645:
2640:
2635:
2627:CDK inhibitors
2623:
2622:
2621:
2616:
2611:
2598:
2597:
2596:
2591:
2586:
2574:
2558:
2538:
2526:fusion protein
2521:
2519:
2515:
2514:
2511:
2510:
2508:
2507:
2506:
2505:
2500:
2495:
2490:
2485:
2472:
2471:
2470:
2469:
2464:
2459:
2442:
2441:
2440:
2439:
2434:
2429:
2424:
2411:
2410:
2409:
2408:
2403:
2398:
2393:
2388:
2383:
2378:
2365:
2364:
2358:
2346:
2345:
2344:
2343:
2338:
2333:
2328:
2323:
2318:
2313:
2300:
2298:
2292:
2291:
2289:
2288:
2267:
2266:
2265:(VEGFR, EGFR).
2221:
2220:
2219:
2218:
2213:
2208:
2195:
2194:
2193:
2192:
2187:
2182:
2177:
2172:
2167:
2162:
2157:
2152:
2147:
2142:
2137:
2132:
2119:
2118:
2102:
2096:
2091:
2086:
2081:
2076:
2071:
2066:
2046:
2045:
2044:
2043:
2038:
2033:
2023:HER1/EGFR and
2019:
2018:
2012:
2007:
2002:
1997:
1992:
1987:
1982:
1977:
1972:
1967:
1962:
1957:
1941:
1939:
1930:
1923:
1922:
1919:
1918:
1916:
1915:
1910:
1905:
1900:
1895:
1890:
1885:
1880:
1875:
1870:
1865:
1860:
1855:
1850:
1845:
1840:
1835:
1830:
1825:
1820:
1815:
1810:
1805:
1800:
1795:
1790:
1785:
1780:
1775:
1770:
1765:
1760:
1755:
1750:
1745:
1740:
1735:
1730:
1725:
1720:
1715:
1710:
1705:
1702:+hyaluronidase
1695:
1689:
1687:
1683:
1682:
1680:
1679:
1662:
1661:
1635:
1630:
1625:
1620:
1615:
1610:
1576:
1574:
1564:
1563:
1561:
1560:
1548:
1542:
1530:
1528:
1524:
1523:
1521:
1520:
1514:
1509:
1506:+hyaluronidase
1489:
1483:
1467:
1465:
1456:
1446:
1445:
1429:
1428:
1421:
1414:
1406:
1400:
1399:
1387:
1375:
1363:
1348:
1347:External links
1345:
1342:
1341:
1282:
1252:
1205:
1179:
1130:
1100:
1075:
1045:
1040:Google Patents
1026:
955:
928:
899:
887:. 27 June 2024
862:
833:
805:
777:
776:
774:
771:
759:gastric cancer
750:
747:
722:
719:
717:
714:
695:
692:
682:
679:
660:bladder cancer
643:
640:
592:
591:
589:
588:
585:
583:
580:
572:
571:
570:
567:
566:
564:
563:
560:
552:
551:
550:
547:
546:
544:
543:
535:
533:
525:
524:
518:
512:
511:
508:
502:
496:
490:
485:
479:
478:
474:
473:
463:
455:
454:
452:
451:
438:
436:
423:
422:
420:
419:
411:
409:
403:
402:
400:
399:
391:
389:
383:
382:
380:
379:
371:
369:
363:
362:
360:
359:
351:
349:
343:
342:
340:
339:
331:
329:
323:
322:
320:
319:
311:
309:
300:
299:
297:
296:
288:
286:
275:
274:
272:
271:
263:
261:
255:
254:
252:
251:
248:
240:
239:
238:
235:
234:
230:
229:
227:
226:
217:
205:
193:
191:
185:
184:
180:
179:
177:
176:
163:
161:
155:
154:
151:
145:
144:
139:
137:administration
131:
130:
128:
127:
109:
107:
101:
100:
93:
87:
86:
79:
69:
68:
65:
61:
60:
57:
51:
50:
46:
45:
26:
9:
6:
4:
3:
2:
2897:
2886:
2883:
2881:
2878:
2876:
2873:
2871:
2868:
2866:
2863:
2862:
2860:
2850:
2840:
2839:
2836:
2823:
2820:
2818:
2815:
2813:
2810:
2808:
2805:
2803:
2800:
2798:
2795:
2793:
2790:
2788:
2785:
2783:
2780:
2778:
2775:
2773:
2770:
2768:
2765:
2763:
2760:
2758:
2755:
2753:
2750:
2748:
2745:
2743:
2740:
2738:
2737:Larotrectinib
2735:
2733:
2730:
2728:
2725:
2723:
2720:
2718:
2715:
2713:
2710:
2706:
2703:
2701:
2698:
2696:
2693:
2691:
2688:
2686:
2683:
2682:
2681:
2678:
2674:
2671:
2669:
2666:
2665:
2664:
2662:
2658:
2654:
2651:
2649:
2646:
2644:
2641:
2639:
2636:
2634:
2631:
2630:
2629:
2628:
2624:
2620:
2617:
2615:
2612:
2610:
2607:
2606:
2605:
2603:
2599:
2595:
2592:
2590:
2589:Ridaforolimus
2587:
2585:
2582:
2581:
2580:
2579:
2575:
2572:
2568:
2567:
2563:
2559:
2556:
2552:
2551:
2547:
2543:
2539:
2536:
2532:
2531:
2527:
2523:
2522:
2520:
2516:
2504:
2501:
2499:
2498:Pirtobrutinib
2496:
2494:
2493:Orelabrutinib
2491:
2489:
2486:
2484:
2483:Acalabrutinib
2481:
2480:
2479:
2478:
2474:
2473:
2468:
2465:
2463:
2460:
2458:
2455:
2454:
2453:
2452:
2448:
2444:
2443:
2438:
2435:
2433:
2430:
2428:
2425:
2423:
2420:
2419:
2418:
2417:
2413:
2412:
2407:
2404:
2402:
2399:
2397:
2394:
2392:
2389:
2387:
2384:
2382:
2379:
2377:
2374:
2373:
2372:
2371:
2367:
2366:
2362:
2359:
2357:
2353:
2352:
2348:
2347:
2342:
2339:
2337:
2334:
2332:
2329:
2327:
2324:
2322:
2319:
2317:
2314:
2312:
2309:
2308:
2307:
2306:
2302:
2301:
2299:
2297:
2293:
2286:
2282:
2278:
2275:
2273:
2269:
2268:
2264:
2260:
2259:Selpercatinib
2256:
2255:Repotrectinib
2252:
2248:
2244:
2243:Larotrectinib
2240:
2236:
2232:
2229:
2227:
2223:
2222:
2217:
2214:
2212:
2209:
2207:
2204:
2203:
2202:
2201:
2197:
2196:
2191:
2188:
2186:
2183:
2181:
2178:
2176:
2173:
2171:
2168:
2166:
2163:
2161:
2158:
2156:
2153:
2151:
2148:
2146:
2143:
2141:
2138:
2136:
2133:
2131:
2128:
2127:
2126:
2125:
2121:
2120:
2116:
2112:
2108:
2107:
2103:
2100:
2097:
2095:
2092:
2090:
2087:
2085:
2082:
2080:
2077:
2075:
2072:
2070:
2067:
2065:
2061:
2060:
2056:
2051:
2050:RTK class III
2048:
2047:
2042:
2039:
2037:
2034:
2032:
2029:
2028:
2027:
2026:
2021:
2020:
2016:
2013:
2011:
2008:
2006:
2003:
2001:
1998:
1996:
1993:
1991:
1988:
1986:
1983:
1981:
1978:
1976:
1973:
1971:
1968:
1966:
1963:
1961:
1958:
1956:
1952:
1951:
1946:
1943:
1942:
1940:
1938:
1934:
1931:
1928:
1924:
1914:
1911:
1909:
1906:
1904:
1901:
1899:
1896:
1894:
1891:
1889:
1886:
1884:
1881:
1879:
1876:
1874:
1871:
1869:
1866:
1864:
1861:
1859:
1856:
1854:
1851:
1849:
1846:
1844:
1841:
1839:
1836:
1834:
1833:Pembrolizumab
1831:
1829:
1826:
1824:
1821:
1819:
1816:
1814:
1811:
1809:
1806:
1804:
1801:
1799:
1798:Mogamulizumab
1796:
1794:
1791:
1789:
1786:
1784:
1781:
1779:
1776:
1774:
1771:
1769:
1766:
1764:
1761:
1759:
1756:
1754:
1751:
1749:
1746:
1744:
1741:
1739:
1736:
1734:
1731:
1729:
1726:
1724:
1721:
1719:
1716:
1714:
1711:
1709:
1706:
1703:
1699:
1696:
1694:
1691:
1690:
1688:
1684:
1677:
1673:
1672:
1667:
1664:
1663:
1659:
1655:
1654:
1649:
1645:
1644:
1639:
1636:
1634:
1631:
1629:
1626:
1624:
1621:
1619:
1618:Mosunetuzumab
1616:
1614:
1611:
1609:
1605:
1604:
1599:
1598:Mosunetuzumab
1595:
1591:
1587:
1586:
1581:
1578:
1577:
1575:
1573:
1569:
1565:
1558:
1554:
1553:
1549:
1546:
1543:
1541:
1537:
1536:
1532:
1531:
1529:
1525:
1518:
1515:
1513:
1510:
1507:
1503:
1499:
1495:
1494:
1490:
1487:
1484:
1482:
1478:
1477:
1472:
1469:
1468:
1466:
1464:
1460:
1457:
1454:
1451:
1447:
1442:
1438:
1434:
1427:
1422:
1420:
1415:
1413:
1408:
1407:
1404:
1398:
1394:
1393:
1388:
1386:
1382:
1381:
1376:
1374:
1370:
1369:
1364:
1362:
1357:
1356:
1351:
1350:
1337:
1333:
1329:
1325:
1320:
1315:
1310:
1305:
1301:
1297:
1293:
1286:
1270:
1266:
1262:
1256:
1249:
1248:public domain
1230:
1226:
1224:
1218:
1212:
1210:
1193:
1189:
1183:
1176:
1175:public domain
1157:
1153:
1147:
1145:
1143:
1141:
1139:
1137:
1135:
1118:
1116:
1110:
1104:
1096:
1094:
1088:
1082:
1080:
1063:
1059:
1055:
1049:
1041:
1037:
1030:
1023:
1022:public domain
1004:
1000:
998:
992:
986:
984:
982:
980:
978:
976:
974:
972:
970:
968:
966:
964:
962:
960:
951:
947:
943:
937:
935:
933:
916:
912:
906:
904:
886:
884:
879:
873:
871:
869:
867:
850:
846:
840:
838:
821:
820:
819:Health Canada
815:
809:
794:
793:
792:Health Canada
788:
782:
778:
770:
768:
764:
760:
756:
746:
743:
739:
734:
732:
728:
713:
709:
706:
700:
691:
687:
678:
675:
671:
669:
665:
661:
657:
652:
649:
639:
637:
633:
629:
626:
622:
618:
614:
610:
606:
602:
598:
584:
579:
578:
575:
568:
559:
558:
555:
548:
541:
537:
536:
534:
531:
526:
519:
517:
513:
486:
484:
480:
475:
471:
467:
464:
462:
460:ECHA InfoCard
456:
448:
444:
440:
439:
437:
428:
424:
417:
416:ChEMBL3545376
413:
412:
410:
408:
404:
397:
393:
392:
390:
388:
384:
377:
373:
372:
370:
368:
364:
357:
353:
352:
350:
348:
344:
337:
333:
332:
330:
328:
324:
317:
313:
312:
310:
303:PubChem
301:
294:
290:
289:
287:
280:
276:
269:
265:
264:
262:
260:
256:
247:
246:
243:
236:
231:
225: Rx-only
218:
216:
206:
204:
195:
194:
192:
190:
186:
181:
173:
168:
165:
164:
162:
160:
156:
152:
150:
146:
143:
140:
138:
132:
125:
120:
111:
110:
108:
106:
102:
98:
94:
92:
88:
84:
80:
78:
74:
70:
66:
62:
58:
56:
52:
49:Clinical data
47:
43:
38:
30:
19:
2885:Quinoxalines
2777:Pexidartinib
2762:Odronextamab
2732:Gilteritinib
2726:
2712:Cabozantinib
2659:
2625:
2600:
2594:Temsirolimus
2576:
2560:
2542:proapoptotic
2540:
2524:
2503:Zanubrutinib
2475:
2445:
2414:
2391:Lestaurtinib
2370:Janus kinase
2368:
2349:
2303:
2296:Non-receptor
2277:Cabozantinib
2270:
2239:Infigratinib
2224:
2198:
2140:Fruquintinib
2122:
2115:Gilteritinib
2111:Lestaurtinib
2104:
2053:
2022:
1995:Mobocertinib
1960:Aumolertinib
1948:
1913:Tremelimumab
1898:Tislelizumab
1853:Retifanlimab
1728:Blinatumomab
1698:Atezolizumab
1669:
1651:
1641:
1623:Obinutuzumab
1601:
1583:
1550:
1533:
1491:
1474:
1390:
1378:
1366:
1353:
1299:
1295:
1285:
1273:. Retrieved
1264:
1255:
1233:. Retrieved
1220:
1196:. Retrieved
1191:
1182:
1160:. Retrieved
1155:
1123:15 September
1121:. Retrieved
1112:
1103:
1090:
1066:. Retrieved
1057:
1048:
1039:
1029:
1007:. Retrieved
994:
945:
919:. Retrieved
914:
889:. Retrieved
881:
853:. Retrieved
848:
824:. Retrieved
817:
808:
796:. Retrieved
790:
781:
752:
735:
724:
721:Legal status
710:
701:
697:
688:
684:
681:Side effects
676:
672:
653:
645:
642:Medical uses
634:for further
621:angiogenesis
600:
596:
595:
268:1346242-81-6
189:Legal status
183:Legal status
105:License data
67:JNJ-42756493
29:
2807:Tebentafusp
2787:Regorafenib
2782:Quizartinib
2772:Pemigatinib
2752:Midostaurin
2727:Erdafitinib
2722:Entrectinib
2705:Parsaclisib
2653:Trilaciclib
2643:Palbociclib
2638:Dalpiciclib
2633:Abemaciclib
2535:Aflibercept
2462:Entrectinib
2432:Selumetinib
2427:Cobimetinib
2422:Binimetinib
2406:Ruxolitinib
2396:Momelotinib
2376:Baricitinib
2274:inhibitors:
2251:Pralsetinib
2247:Pemigatinib
2235:Futibatinib
2231:Entrectinib
2228:inhibitors:
2160:Regorafenib
2064:Avapritinib
2010:Rociletinib
2005:Osimertinib
1970:Dacomitinib
1908:Toripalimab
1893:Teclistamab
1883:Talquetamab
1878:Tafasitamab
1873:Sugemalimab
1868:Serplulimab
1858:Sabatolimab
1848:Ramucirumab
1843:Prolgolimab
1813:Necitumumab
1768:Epcoritamab
1748:Dostarlimab
1738:Daratumumab
1693:Amivantamab
1658:Alemtuzumab
1648:Brentuximab
1638:Tositumomab
1613:Ibritumomab
1594:Elranatamab
1557:Bevacizumab
1545:Edrecolomab
1540:Catumaxomab
1502:Trastuzumab
1486:Panitumumab
1392:NCT01703481
1380:NCT03473743
1368:NCT02365597
1355:NCT03390504
1235:24 November
1068:24 November
636:development
597:Erdafitinib
523: g·mol
466:100.235.008
233:Identifiers
124:Erdafitinib
91:MedlinePlus
64:Other names
55:Trade names
34:Erdafitinib
2859:Categories
2822:Venetoclax
2817:Vandetanib
2792:Ripretinib
2757:Nintedanib
2742:Lenvatinib
2717:Capmatinib
2700:Idelalisib
2690:Copanlisib
2663:inhibitors
2648:Ribociclib
2619:Vismodegib
2604:inhibitors
2584:Everolimus
2550:prohibitin
2467:Lorlatinib
2457:Crizotinib
2437:Trametinib
2401:Pacritinib
2386:Filgotinib
2381:Fedratinib
2285:Crizotinib
2281:Capmatinib
2263:Vandetanib
2211:Brigatinib
2190:Vandetanib
2150:Nintedanib
2145:Lenvatinib
2084:Ripretinib
2015:Vandetanib
1990:Lazertinib
1965:Brigatinib
1888:Tarlatamab
1823:Olaratumab
1783:Isatuximab
1778:Ipilimumab
1758:Elotuzumab
1753:Durvalumab
1733:Cemiplimab
1723:Bermekimab
1713:Axatilimab
1628:Ofatumumab
1608:Glofitamab
1590:Glofitamab
1498:Pertuzumab
1319:10854/7722
773:References
628:discovered
528:3D model (
516:Molar mass
376:890E37NHMV
347:ChemSpider
259:CAS Number
242:IUPAC name
149:Drug class
2880:Pyrazoles
2812:Tepotinib
2802:Sunitinib
2797:Sorafenib
2767:Pazopanib
2747:Masitinib
2695:Duvelisib
2685:Alpelisib
2673:Sotorasib
2668:Adagrasib
2614:Sonidegib
2609:Glasdegib
2555:Adipotide
2488:Ibrutinib
2361:Dasatinib
2356:Bosutinib
2341:Radotinib
2336:Ponatinib
2331:Nilotinib
2321:Dasatinib
2316:Bosutinib
2311:Asciminib
2279:(VEGFR),
2237:(FGFR2),
2216:Ceritinib
2206:Alectinib
2185:Toceranib
2180:Tivozanib
2175:Sunitinib
2170:Sorafenib
2165:Semaxanib
2155:Pazopanib
2135:Cediranib
2099:Toceranib
2094:Sunitinib
2089:Sorafenib
2079:Pazopanib
2074:Masitinib
2041:Tucatinib
2036:Neratinib
2031:Lapatinib
2000:Olmutinib
1980:Gefitinib
1975:Erlotinib
1950:HER1/EGFR
1818:Nivolumab
1808:Naxitamab
1633:Rituximab
1481:Cetuximab
1476:HER1/EGFR
1336:155089088
1198:26 August
921:27 August
674:therapy.
648:indicated
316:347828443
135:Routes of
83:Monograph
77:Drugs.com
2849:Medicine
2602:hedgehog
2564:against
2562:exotoxin
2528:against
2477:Bruton's
2326:Imatinib
2249:(FGFR),
2245:(NTRK),
2130:Axitinib
2069:Axitinib
2025:HER2/neu
1985:Icotinib
1955:Afatinib
1929:("-nib")
1708:Avelumab
1580:lymphoid
1572:lymphoma
1568:Leukemia
1493:HER2/neu
1455:("-mab")
1328:31088831
1269:Archived
1229:Archived
1062:Archived
1003:Archived
950:Archived
849:DailyMed
749:Research
603:, is an
601:Balversa
356:35308353
327:DrugBank
293:67462786
159:ATC code
142:By mouth
119:DailyMed
59:Balversa
18:Balversa
2305:bcr-abl
1666:myeloid
1275:12 July
1192:OncLive
1162:9 March
891:29 June
855:29 June
740:of the
694:History
521:446.555
483:Formula
336:DB12147
279:PubChem
201::
175:)
169: (
167:L01EN01
121::
97:a619031
2835:Portal
1552:VEGF-A
1334:
1326:
1009:13 May
826:29 May
798:29 May
765:, and
554:SMILES
407:ChEMBL
396:D10927
215:℞-only
213:
203:℞-only
117:
2546:ANXA2
2518:Other
2416:MAP2K
2287:(ALK)
2272:c-MET
2124:VEGFR
2059:PDGFR
2055:C-kit
1686:Other
1535:EpCAM
1332:S2CID
1225:(FDA)
1221:U.S.
1117:(FDA)
1113:U.S.
1095:(FDA)
1091:U.S.
999:(FDA)
995:U.S.
885:(EMA)
574:InChI
530:JSmol
2661:KRAS
2566:IL-2
2548:and
2530:VEGF
2447:EML4
2106:FLT3
2057:and
1945:ErbB
1671:CD33
1653:CD52
1643:CD30
1603:CD20
1471:ErbB
1324:PMID
1277:2024
1237:2019
1200:2024
1164:2024
1125:2020
1070:2019
1011:2019
923:2024
893:2024
857:2024
828:2022
800:2022
387:KEGG
367:UNII
73:AHFS
2451:ALK
2351:Src
2226:RET
2200:ALK
1650:),
1640:),
1600:),
1585:CD3
1441:L01
1314:hdl
1304:doi
432:EPA
306:SID
283:CID
172:WHO
2861::
2283:,
2253:,
2241:,
2113:,
2052::
1947::
1668::
1596:,
1592:,
1582::
1500:,
1473::
1450:CI
1435:/
1330:.
1322:.
1312:.
1300:25
1298:.
1294:.
1263:.
1219:.
1208:^
1190:.
1154:.
1133:^
1111:.
1089:.
1078:^
1056:.
1038:.
993:.
958:^
944:.
931:^
913:.
902:^
880:.
865:^
847:.
836:^
816:.
789:.
769:.
761:,
757:,
733:.
670:.
638:.
497:30
491:25
221:EU
209:US
198:CA
114:US
2837::
2573:)
2569:(
2557:)
2553:(
2537:)
2533:(
2449:-
2363:)
2354:(
2109:(
2101:)
2062:(
2017:)
1953:(
1704:)
1700:(
1678:)
1674:(
1660:)
1656:(
1646:(
1606:(
1588:(
1570:/
1559:)
1555:(
1547:)
1538:(
1519:)
1508:)
1504:(
1496:(
1488:)
1479:(
1443:)
1439:(
1425:e
1418:t
1411:v
1338:.
1316::
1306::
1279:.
1250:.
1239:.
1202:.
1177:.
1166:.
1127:.
1097:.
1072:.
1042:.
1024:.
1013:.
925:.
895:.
859:.
830:.
802:.
532:)
509:2
506:O
503:6
500:N
494:H
488:C
434:)
430:(
223::
211::
75:/
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.