127:) and as micelles, below the CMC SDS occurs only as monomers in aqueous solutions. At the critical micellar concentration, a micelle consists of about 62 SDS molecules. However, only SDS monomers bind to proteins via hydrophobic interactions, whereas the SDS micelles are anionic on the outside and do not adsorb any protein. SDS is amphipathic in nature, which allows it to unfold both polar and nonpolar sections of protein structure. In SDS concentrations above 0.1 millimolar, the unfolding of proteins begins, and above 1 mM, most proteins are denatured. Due to the strong denaturing effect of SDS and the subsequent dissociation of protein complexes,
406:
behind the proteins (as initial trailing ions), whereas in the comparatively basic separating gel both ions migrate in front of the proteins. The pH gradient between the stacking and separation gel buffers leads to a stacking effect at the border of the stacking gel to the separation gel, since the glycinate partially loses its slowing positive charges as the pH increases and then, as the former trailing ion, overtakes the proteins and becomes a leading ion, which causes the bands of the different proteins (visible after a staining) to become narrower and sharper - the stacking effect. For the separation of smaller proteins and peptides, the TRIS-
572:
as the distance migrated by the protein band divided by the distance migrated by the buffer front. The distances are each measured from the beginning of the separation gel. The migration of the buffer front roughly corresponds to the migration of the dye contained in the sample buffer. The Rf's of the size marker are plotted semi-logarithmically against their known molecular weights. By comparison with the linear part of the generated graph or by a regression analysis, the molecular weight of an unknown protein can be determined by its relative mobility.
20:
268:
179:
334:
80:
112:, masking the protein's intrinsic charge and conferring them very similar charge-to-mass ratios. The intrinsic charges of the proteins are negligible in comparison to the SDS loading, and the positive charges are also greatly reduced in the basic pH range of a separating gel. Upon application of a constant electric field, the proteins migrate towards the anode, each with a different speed, depending on their mass. This simple procedure allows precise protein separation by mass.
251:) and have a nearly neutral pH, they can be stored for several weeks. The more neutral pH slows the hydrolysis and thus the decomposition of the polyacrylamide. Furthermore, there are fewer acrylamide-modified cysteines in the proteins. Due to the constant pH in collecting and separating gel there is no stacking effect. Proteins in BisTris gels can not be stained with ruthenium complexes. This gel system has a comparatively large separation range, which can be varied by using
72:
212:(APS) the polymerisation is started. The solution is then poured between the glass plates without creating bubbles. Depending on the amount of catalyst and radical starter and depending on the temperature, the polymerisation lasts between a quarter of an hour and several hours. The lower gel (separating gel) is poured first and covered with a few drops of a barely water-soluble alcohol (usually buffer-saturated butanol or isopropanol), which eliminates bubbles from the
135:(e.g. -S-S- linkages) and the SDS-resistant protein complexes, which are stable even in the presence of SDS (the latter, however, only at room temperature). To denature the SDS-resistant complexes a high activation energy is required, which is achieved by heating. SDS resistance is based on a metastability of the protein fold. Although the native, fully folded, SDS-resistant protein does not have sufficient stability in the presence of SDS, the
538:
187:
419:
345:
564:
361:. The gel acts like a sieve. Small proteins migrate relatively easily through the mesh of the gel, while larger proteins are more likely to be retained and thereby migrate more slowly through the gel, thereby allowing proteins to be separated by molecular size. The electrophoresis lasts between half an hour to several hours depending on the voltage and length of gel used.
224:. After addition of APS and TEMED to the stacking gel solution, it is poured on top of the solid separation gel. Afterwards, a suitable sample comb is inserted between the glass plates without creating bubbles. The sample comb is carefully pulled out after polymerisation, leaving pockets for the sample application. For later use of proteins for
579:) can lead to an overestimation of the molecular weight or even not migrate into the gel at all, because they move slower in the electrophoresis due to the positive charges or even to the opposite direction. On the other hand, many acidic amino acids can lead to accelerated migration of a protein and an underestimation of its molecular mass.
204:
which temporarily seals the otherwise open underside of the glass plates with the two spacers. For the gel solution, acrylamide is mixed as gel-former (usually 4% V/V in the stacking gel and 10-12 % in the separating gel), methylenebisacrylamide as a cross-linker, stacking or separating gel buffer, water and SDS. By adding the catalyst
545:
After protein staining and documentation of the banding pattern, the polyacrylamide gel can be dried for archival storage. Proteins can be extracted from it at a later date. The gel is either placed in a drying frame (with or without the use of heat) or in a vacuum dryer. The drying frame consists of
373:
The most commonly used method is the discontinuous SDS-PAGE. In this method, the proteins migrate first into a collecting gel with neutral pH, in which they are concentrated and then they migrate into a separating gel with basic pH, in which the actual separation takes place. Stacking and separating
203:
in a mold consisting of two sealed glass plates with spacers between the glass plates. In a typical mini-gel setting, the spacers have a thickness of 0.75 mm or 1.5 mm, which determines the loading capacity of the gel. For pouring the gel solution, the plates are usually clamped in a stand
571:
For a more accurate determination of the molecular weight, the relative migration distances of the individual protein bands are measured in the separating gel. The measurements are usually performed in triplicate for increased accuracy. The relative mobility (called Rf value or Rm value) is defined
554:
solution are added. Then a second wet cellophane film is applied bubble-free, the second frame part is put on top and the frame is sealed with clips. The removal of the air bubbles avoids a fragmentation of the gel during drying. The water evaporates through the cellophane film. In contrast to the
512:
when sufficient amounts of detergent are not present. This precipitation manifests itself for membrane proteins in a SDS-PAGE in "tailing" above the band of the transmembrane protein. In this case, more SDS can be used (by using more or more concentrated sample buffer) and the amount of protein in
324:
is usually loaded onto the gel. This consists of proteins of known sizes and thereby allows the estimation (with an error of ± 10%) of the sizes of the proteins in the actual samples, which migrate in parallel in different tracks of the gel. The size marker is often pipetted into the first or last
405:
form, at high pH the glycines lose positive charges and become predominantly anionic. In the collection gel, the smaller, negatively charged chloride ions migrate in front of the proteins (as leading ions) and the slightly larger, negatively and partially positively charged glycinate ions migrate
470:
staining, gel is fixed in a 50% ethanol 10% glacial acetic acid solution for 1 hr. Then the solution is changed for fresh one and after 1 to 12 hrs gel is changed to a staining solution (50% methanol, 10% glacial acetic acid, 0.1% coomassie brilliant blue) followed by destaining changing several
794:
improved the separation. The discontinuous electrophoresis of 1964 by L. Ornstein and B. J. Davis made it possible to improve the separation by the stacking effect. The use of cross-linked polyacrylamide hydrogels, in contrast to the previously used paper discs or starch gels, provided a higher
369:
to the sample buffer. Due to the relatively small molecule size of bromophenol blue, it migrates faster than proteins. By optical control of the migrating colored band, the electrophoresis can be stopped before the dye and also the samples have completely migrated through the gel and leave it.
364:
The fastest-migrating proteins (with a molecular weight of less than 5 kDa) form the buffer front together with the anionic components of the electrophoresis buffer, which also migrate through the gel. The area of the buffer front is made visible by adding the comparatively small, anionic dye
520:
A low contrast (as in the marker lane of the image) between bands within a lane indicates either the presence of many proteins (low purity) or, if using purified proteins and a low contrast occurs only below one band, it indicates a proteolytic degradation of the protein, which first causes
1583:
Wiltfang, Jens; Arold, Norbert; Neuhoff, Volker (1991). "A new multiphasic buffer system for sodium dodecyl sulfate-polyacrylamide gel electrophoresis of proteins and peptides with molecular masses 100 000-1000, and their detection with picomolar sensitivity".
587:
The SDS-PAGE in combination with a protein stain is widely used in biochemistry for the quick and exact separation and subsequent analysis of proteins. It has comparatively low instrument and reagent costs and is an easy-to-use method. Because of its low
62:
gel eliminates the influence of structure and charge, and proteins are separated by differences in their size. At least up to 2012, the publication describing it was the most frequently cited paper by a single author, and the second most cited overall.
275:
During sample preparation, the sample buffer, and thus SDS, is added in excess to the proteins, and the sample is then heated to 95 °C for five minutes, or alternatively 70 °C for ten minutes. Heating disrupts the
714:
While being one of the more precise and low-cost protein separation and analysis methods, the SDS-PAGE denatures proteins. Where non-denaturing conditions are necessary, proteins are separated by a native PAGE or different
422:
Coomassie-stained 10% Tris/Tricine gel. In the left lane, a molecular weight size marker was used to estimate the size (from top to bottom: 66, 45, 35, 24, 18 and 9 kDa). In the remaining lanes purified yeast proteins were
685:
is used if native protein folding is to be maintained. For separation of membrane proteins, BAC-PAGE or CTAB-PAGE may be used as an alternative to SDS-PAGE. For electrophoretic separation of larger protein complexes,
190:
Polymerised separating and stacking gel before removing the sample comb (white) between the spacers (black), in the stacking gel are small amounts of bromophenol blue for improved visibility, the separating gel is
348:
Electrophoresis chamber after an hour of electrophoresis at 80 Volts. In the first and the last two wells loaded, a commercial protein ladder was applied. The other loaded wells contain protein samples coated in
316:
are added to the sample buffer. After cooling to room temperature, each sample is pipetted into its own well in the gel, which was previously immersed in electrophoresis buffer in the electrophoresis apparatus.
91:
method that allows protein separation by mass. The medium (also referred to as ′matrix′) is a polyacrylamide-based discontinuous gel. The polyacrylamide-gel is typically sandwiched between two glass plates in a
1319:
Akin, Dianne T.; Shapira, Raymond; Kinkade, Joseph M. (1985). "The determination of molecular weights of biologically active proteins by cetyltrimethylammonium bromide-polyacrylamide gel electrophoresis".
516:
An overloading of the gel with a soluble protein creates a semicircular band of this protein (e. g. in the marker lane of the image at 66 kDa), allowing other proteins with similar molecular weights to be
567:
The proteins of the size marker (black X) show an approximately straight line in the representation of log M over Rf. The molecular weight of the unknown protein (red X) can be determined on the y-axis.
795:
stability of the gel and no microbial decomposition. The denaturing effect of SDS in continuous polyacrylamide gels and the consequent improvement in resolution was first described in 1965 by
790:
for the discovery of the principle of electrophoresis as the migration of charged and dissolved atoms or molecules in an electric field. The use of a solid matrix (initially paper discs) in a
1079:
Staikos, Georgios; Dondos, Anastasios (2009). "Study of the sodium dodecyl sulphate–protein complexes: evidence of their wormlike conformation by treating them as random coil polymers".
2222:
Moritz, Christian P.; Marz, Sabrina X.; Reiss, Ralph; Schulenborg, Thomas; Friauf, Eckhard (February 2014). "Epicocconone staining: a powerful loading control for
Western blots".
248:
239:, gradient gels with a gradient of acrylamide (usually from 4 to 12%) can be cast, which have a larger separation range of the molecular masses. Commercial gel systems (so-called
357:(usually around 100 V, 10-20 V per cm gel length) is applied, which causes a migration of negatively charged molecules through the gel in the direction of the positively charged
337:
Electrophoresis chamber after a few minutes of electrophoresis. In the first pocket a size marker was applied with bromophenol blue, in the other pockets, the samples were added
1824:
Schägger, Hermann; von Jagow, Gebhard (1987). "Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa".
1635:
Moebius, Jan; Denker, Katrin; Sickmann, Albert (2007). "Ruthenium (II) tris-bathophenanthroline disulfonate is well suitable for Tris-Glycine PAGE but not for Bis-Tris gels".
803:
to separate poliovirus proteins. The current variant of the SDS-PAGE was described in 1970 by Ulrich K. Laemmli and initially used to characterise the proteins in the head of
1155:
Turro, Nicholas J.; Yekta, Ahmad (1978). "Luminescent probes for detergent solutions. A simple procedure for determination of the mean aggregation number of micelles".
247:
with a pH value between 6.4 and 7.2 both in the stacking gel and in the separating gel. These gels are delivered cast and ready-to-use. Since they use only one buffer (
353:
For separation, the denatured samples are loaded onto a gel of polyacrylamide, which is placed in an electrophoresis buffer with suitable electrolytes. Thereafter, a
2267:
Advanced
Fluorescence Reporters in Chemistry and Biology III: Applications in Sensing and Imaging Band 3 von Advanced Fluorescence Reporters in Chemistry and Biology
1540:
Hachmann, John P.; Amshey, Joseph W. (2005). "Models of protein modification in Tris–glycine and neutral pH Bis–Tris gels during electrophoresis: Effect of gel pH".
1907:
Wilson, CM (1979). "Studies and critique of Amido Black 10B, Coomassie Blue R, and Fast Green FCF as stains for proteins after polyacrylamide gel electrophoresis".
1239:"Structural basis of protein kinetic stability: resistance to sodium dodecyl sulfate suggests a central role for rigidity and a bias toward beta-sheet structure"
1867:
Fazekas de St. Groth, S.; Webster, R. G.; Datyner, A. (1963). "Two new staining procedures for quantitative estimation of proteins on electrophoretic strips".
2909:"Protein mapping by combined isoelectric focusing and electrophoresis of mouse tissues. A novel approach to testing for induced point mutations in mammals"
525:
The documentation of the banding pattern is usually done by photographing or scanning. For a subsequent recovery of the molecules in individual bands, a
2003:
R. C. Switzer, C. R. Merril, S. Shifrin (September 1979), "A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels",
912:
886:
427:
At the end of the electrophoretic separation, all proteins are sorted by size and can then be analyzed by other methods, e. g. protein staining such as
796:
455:. The fluorescent dyes have a comparatively higher linearity between protein quantity and color intensity of about three orders of magnitude above the
139:
of denaturation at room temperature occurs slowly. Stable protein complexes are characterised not only by SDS resistance but also by stability against
100:(in glass cylinders) were used historically, they were rapidly made obsolete with the invention of the more convenient slab gels. In addition, SDS (
2792:
2033:
236:
195:
When using different buffers in the gel (discontinuous gel electrophoresis), the gels are made up to one day prior to electrophoresis, so that the
650:
1944:"Trace polypeptides in cellular extracts and human body fluids detected by two-dimensional electrophoresis and a highly sensitive silver stain"
1223:
592:, it is mostly used for analytical purposes and less for preparative purposes, especially when larger amounts of a protein are to be isolated.
463:, a subsequent protein staining is omitted if it was added to the gel solution and the gel was irradiated with UV light after electrophoresis.
2082:
Rabilloud, T.; et al. (1988). "Improvement and simplification of low-background silver staining of proteins by using sodium dithionite".
575:
Bands of proteins with glycosylations can be blurred, as glycosylation is often heterogenous. Proteins with many basic amino acids (e. g.
97:
981:"Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes"
123:(CMC). Above the critical micellar concentration of 7 to 10 millimolar in solutions, the SDS simultaneously occurs as single molecules (
2678:"An equation to estimate the difference between theoretically predicted and SDS PAGE-displayed molecular weights for an acidic peptide"
93:
2955:
Vandooren J, Geurts N, Martens E, Van den Steen PE, Opdenakker G (2013). "Zymography methods for visualizing hydrolytic enzymes".
3057:
3032:
3007:
2660:
2596:
2569:
2514:
2487:
2274:
2190:
1758:
1131:
220:
oxygen. After the polymerisation of the separating gel, the alcohol is discarded and the residual alcohol is removed with
1408:"16-BAC/SDS–PAGE: A Two-Dimensional Gel Electrophoresis System Suitable for the Separation of Integral Membrane Proteins"
674:
410:
buffer system of Schägger and von Jagow is used due to the higher spread of the proteins in the range of 0.5 to 50 kDa.
641:. In the HIV test, HIV proteins are separated by SDS-PAGE and subsequently detected by Western Blot with HIV-specific
607:) or to a change in the binding of a detection antibody used in the western blot (i.e. a band disappears or appears).
2362:
1199:
1055:
1482:"2-dimensional resolution of plasma proteins by combination of polyacrylamide disc and gradient gel electrophoresis"
2047:
Blum, H.; Beier, H.; Gross, H. J. (1987). "Improved silver staining of plant protein, RNA & DNA in PAA gels".
228:, the gels are often prepared the day before electrophoresis to reduce reactions of unpolymerised acrylamide with
618:. In regards to determining the molecular mass of a protein, the SDS-PAGE is a bit more exact than an analytical
600:
120:
2125:
Rabilloud, T. (1992). "A comparison between low background silver diammine and silver nitrate protein stains".
767:
104:) is used. About 1.4 grams of SDS bind to a gram of protein, corresponding to one SDS molecule charges per two
827:
Laemmli, U. K. (1970). "Cleavage of
Structural Proteins during the Assembly of the Head of Bacteriophage T4".
599:
for the determination of the presence of a specific protein in a mixture of proteins - or for the analysis of
735:
309:
166:
The SDS-PAGE method is composed of gel preparation, sample preparation, electrophoresis, protein staining or
39:
2169:
731:
459:(the quantity of protein that can be estimated by color intensity). When using the fluorescent protein dye
321:
2631:
754:. Some historically early and cost effective but crude separation methods usually based upon a series of
739:
687:
614:
of proteins, SDS-PAGE is a widely used method for sample preparation prior to spectrometry, mostly using
200:
2168:
Lelong, C.; Chevallet, M.; Luche, S.; Rabilloud, T. (2009). "Silver
Staining of Proteins in 2DE Gels".
743:
626:
or - ignoring post-translational modifications - a calculation of the protein molecular mass from the
150:
Alternatively, polyacrylamide gel electrophoresis can also be performed with the cationic surfactants
1276:
Buxbaum, Engelbert (2003). "Cationic electrophoresis and electrotransfer of membrane glycoproteins".
521:
degradation bands, and after further degradation produces a homogeneous color ("smear") below a band.
182:
Sample combs with different numbers of pockets, each prong leaves a pocket in the gel when pulled out
3259:
1948:
787:
723:
467:
428:
131:
can generally not be determined with SDS. Exceptions are proteins that are stabilised by covalent
727:
603:. Post-translational modifications of proteins can lead to a different relative mobility (i.e. a
3243:
755:
101:
55:
2613:
2586:
2559:
2504:
2427:"Stain-Free total protein staining is a superior loading control to β-actin for Western blots"
1748:
2786:
2650:
2477:
658:
479:
Protein staining in the gel creates a documentable banding pattern of the various proteins.
3194:
3139:
2689:
1957:
1493:
992:
836:
791:
678:
497:
490:
and adsorb SDS more unevenly at the glycosylations, resulting in broader and blurred bands.
144:
136:
128:
297:
8:
1709:
720:
619:
277:
209:
151:
3198:
3143:
2693:
1961:
1497:
1194:. Tymoczko, John L.; Gatto, Gregory J. Jr.; Stryer, Lubert (Eighth ed.). New York.
996:
840:
673:
SDS-PAGE is the most widely used method for gel electrophoretic separation of proteins.
3163:
3151:
3112:
2980:
2937:
2908:
2883:
2856:
2837:
2769:
2742:
2718:
2677:
2451:
2426:
2343:
Wilson, CM (1983). "Staining of proteins on gels: Comparisons of dyes and procedures".
2320:
2291:
2247:
2204:
2150:
2107:
2064:
1668:
1617:
1517:
1467:
1217:
1104:
868:
771:
555:
drying frame, a vacuum dryer generates a vacuum and heats the gel to about 50 °C.
509:
281:
225:
3217:
3182:
3050:
Protein
Bioseparation Using Ultrafiltration: Theory, Applications And New Developments
2873:
2354:
1980:
1943:
1289:
1015:
980:
938:
3222:
3155:
3130:
Ornstein, L.; Davis, B. J. (1964). "Disc
Electrophoresis –1. Background and Theory".
3104:
3096:
3053:
3028:
3003:
2972:
2929:
2888:
2841:
2829:
2774:
2723:
2705:
2656:
2592:
2565:
2510:
2483:
2456:
2407:
2368:
2358:
2325:
2307:
2270:
2251:
2239:
2196:
2186:
2142:
2099:
2027:
2016:
2012:
1985:
1924:
1920:
1884:
1880:
1849:
1841:
1837:
1754:
1660:
1652:
1609:
1601:
1565:
1557:
1509:
1437:
1429:
1388:
1380:
1345:
1337:
1333:
1301:
1293:
1258:
1205:
1195:
1172:
1137:
1127:
1096:
1061:
1051:
1020:
950:
942:
860:
852:
623:
611:
541:
Two SDS gels after completed separation of the samples and staining in a drying frame
338:
313:
217:
213:
43:
19:
3167:
3116:
2941:
2208:
2154:
2111:
2068:
1672:
1621:
1108:
750:. Single proteins can be isolated from a mixture by affinity chromatography or by a
3212:
3202:
3147:
3086:
2984:
2964:
2921:
2878:
2868:
2819:
2764:
2754:
2713:
2697:
2538:
2446:
2438:
2399:
2388:"Visible fluorescent detection of proteins in polyacrylamide gels without staining"
2350:
2315:
2299:
2231:
2178:
2134:
2091:
2056:
2008:
1975:
1965:
1916:
1876:
1833:
1807:
1782:
1730:
1705:
1697:
1644:
1593:
1549:
1521:
1501:
1463:
1419:
1372:
1329:
1285:
1250:
1164:
1088:
1043:
1010:
1000:
934:
872:
844:
804:
800:
615:
493:
460:
366:
305:
289:
244:
3183:"Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells"
3075:"Turning a PAGE: the overnight sensation of SDS-polycrylamide gel electrophoresis"
2542:
2182:
1786:
976:
751:
747:
699:
456:
440:
398:
88:
1811:
1406:
Hartinger, Joachim; Stenius, Katinka; Högemann, Dagmar; Jahn, Reinhard (1996).
1047:
716:
526:
487:
444:
379:
301:
267:
59:
1701:
1092:
386:(pH 6.8 or pH 8.8). The electrolyte most frequently used is an SDS-containing
178:
3253:
3100:
2759:
2709:
2442:
2403:
2311:
1845:
1656:
1605:
1561:
1553:
1433:
1384:
1341:
1297:
1209:
1176:
1141:
1100:
946:
856:
783:
759:
333:
285:
2138:
2095:
2060:
1597:
3159:
3108:
3000:
Protein
Purification: Principles, High Resolution Methods, and Applications
2976:
2833:
2808:"Western blotting: a powerful staple in scientific and biomedical research"
2778:
2727:
2460:
2411:
2387:
2329:
2243:
2235:
2200:
1970:
1888:
1664:
1648:
1569:
1424:
1407:
1392:
1305:
1262:
1238:
1065:
1038:
1005:
954:
627:
596:
483:
452:
448:
432:
402:
252:
221:
167:
3226:
3207:
3091:
3074:
2933:
2824:
2807:
2372:
2344:
2146:
2103:
1853:
1613:
1513:
1481:
1441:
1349:
1024:
864:
375:
79:
71:
2892:
1376:
763:
682:
646:
638:
589:
24:
2020:
1989:
1928:
1734:
1168:
2968:
2925:
2002:
703:
642:
547:
501:
436:
132:
109:
105:
51:
2701:
1505:
1254:
471:
times a destaining solution of 40% methanol, 10% glacial acetic acid.
451:
stain and SYPRO orange stain, and immunological detection such as the
1721:
John A. Burns, James C. Butler, John T. Moran, George M. Whitesides:
1456:
One-dimensional SDS-polyacrylamide gel electrophoresis (1D SDS-PAGE).
848:
537:
500:, are often composed of the more hydrophobic amino acids, have lower
196:
1124:
Fundamental
Laboratory Approaches for Biochemistry and Biotechnology
2954:
1723:
Selective reduction of disulfides by tris(2-carboxyethyl)phosphine.
634:
551:
395:
383:
229:
140:
1866:
27:
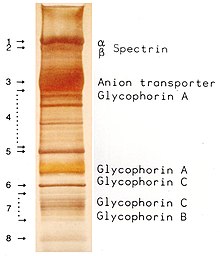
membrane separated by SDS-PAGE according to their molecular masses
1690:
Optimized
Proteome Reduction for Integrative Top–Down Proteomics.
691:
654:
576:
407:
391:
354:
199:
does not lead to a mixing of the buffers. The gel is produced by
124:
116:
47:
2303:
695:
563:
186:
155:
2676:
Guan, Yihong; Zhu, Qinfang; Huang, Delai; et al. (2015).
119:
in aqueous solutions above a certain concentration called the
2857:"High resolution two-dimensional electrophoresis of proteins"
2503:
Burgess, Richard R.; Deutscher, Murray P. (3 November 2009).
2167:
1042:. Methods in Molecular Biology. Vol. 1. pp. 41–56.
505:
418:
358:
293:
205:
2558:
Bonner, Philip L. R.; Hargreaves, Alan J. (24 August 2011).
1405:
2649:
van Venrooij, W. J.; Maini, Ravinder N. (6 December 2012).
649:. SDS-PAGE for proteinuria evaluates the levels of various
387:
256:
2221:
344:
2007:(in German), vol. 98, no. 1, pp. 231–237,
1942:
Merril, C. R.; Switzer, R. C.; Keuren, M. L. Van (1979).
1126:(2nd ed.). Hoboken, NJ: John Wiley & Sons, Inc.
662:
36:
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
3025:
Downstream Processing of Proteins: Methods and Protocols
2591:. Springer Science & Business Media. pp. 243–.
1753:. Springer Science & Business Media. pp. 103–.
633:
In medical diagnostics, SDS-PAGE is used as part of the
292:
can be cleaved by reduction. For this purpose, reducing
2743:"Western blot: technique, theory, and trouble shooting"
2655:. Springer Science & Business Media. pp. 50–.
2553:
2551:
2385:
2283:
1688:
Breyer Woodland, Aleksandar Necakov, Jens R. Coorssen:
595:
Additionally, SDS-PAGE is used in combination with the
401:
system. At neutral pH, glycine predominantly forms the
2805:
2561:
Basic Bioscience Laboratory Techniques: A Pocket Guide
2479:
Cryo-EM Part A: Sample Preparation and Data Collection
1750:
Protein Analysis and Purification: Benchtop Techniques
1715:
508:, and tend to precipitate in aqueous solutions due to
3027:. Springer Science & Business Media. p. 35.
2177:. Methods Mol Biol. Vol. 519. pp. 339–350.
1817:
1634:
1535:
1533:
1531:
925:
Studier, F (2000-12-01). "Slab-gel electrophoresis".
3180:
2548:
2496:
1582:
1399:
2289:
1941:
546:two parts, one of which serves as a base for a wet
2907:
2349:. Methods Enzymol. Vol. 91. pp. 236–47.
1528:
1318:
915:. NZZ Folio, No. 11, 2005. Accessed March 4, 2012.
2854:
2648:
2642:
2529:S. Stamova, I. Michalk, H. Bartsch, M. Bachmann:
2482:. Academic Press. 30 September 2010. p. 28.
2386:Ladner CL, Yang J, Turner RJ, Edwards RA (2004).
1823:
1628:
1576:
1121:
3251:
2675:
2557:
2502:
1072:
2806:Begum H, Murugesan P, Tangutur AD (June 2022).
1479:
1312:
974:
558:
170:and analysis of the generated banding pattern.
58:(SDS, also known as sodium lauryl sulfate) and
46:which is commonly used as a method to separate
2632:"Beginners Guide To Glycosylation Of Proteins"
2578:
2046:
1539:
742:. Proteins can also be separated by size in a
3129:
2948:
1902:
1900:
1898:
1740:
1148:
1078:
822:
820:
3066:
2791:: CS1 maint: DOI inactive as of June 2024 (
2032:: CS1 maint: multiple names: authors list (
1236:
2740:
2669:
1729:1991, Band 56, Nummer 8, S. 2648–2650
1684:
1682:
1356:
1269:
645:of the patient, if they are present in his
3181:Summers DF, Maizel JV, Darnell JE (1965).
2991:
2747:North American Journal of Medical Sciences
2584:
2424:
2336:
1895:
1767:
1222:: CS1 maint: location missing publisher (
1154:
970:
968:
966:
964:
918:
817:
288:and stretching the molecules. Optionally,
3216:
3206:
3090:
3016:
2882:
2872:
2823:
2768:
2758:
2717:
2450:
2319:
2290:Gallagher, Sean; Chakavarti, Deb (2008).
2264:
2258:
2171:Two-Dimensional Electrophoresis Protocols
2124:
2081:
1979:
1969:
1746:
1423:
1014:
1004:
3072:
3041:
2611:
2585:Holtzhauer, Martin (13 September 2006).
2564:. John Wiley & Sons. pp. 140–.
1679:
1473:
1448:
1157:Journal of the American Chemical Society
1031:
913:Interview with Ulrich Lämmli (in German)
562:
550:film to which the gel and a one percent
536:
431:staining (most common and easy to use),
417:
378:size (4-6 % T and 10-20 % T),
343:
332:
266:
185:
177:
78:
70:
50:with molecular masses between 5 and 250
18:
3174:
2998:Janson, Jan-Christer (3 January 2012).
2734:
2652:Manual of Biological Markers of Disease
2523:
2472:
2470:
2265:Demchenko, Aleksandr Petrovich (2011).
1792:
1362:
1275:
1230:
961:
924:
826:
3252:
2997:
2379:
2342:
1906:
1747:Rosenberg, Ian M. (22 December 2006).
1122:Ninfa AJ, Ballou DP, Benore M (2010).
513:the sample application can be reduced.
3022:
2905:
2799:
2741:Mahmood T, Yang PC (September 2012).
2614:"Measuring mobility of protein bands"
2612:Caprette, David R (January 5, 2007).
2588:Basic Methods for the Biochemical Lab
2418:
1037:
447:staining, fluorescent stains such as
262:
216:and protects the gel solution of the
3047:
2467:
1781:Volume 1855, 2019, p. 115–124,
1696:2023, Band 11, Nummer 1, S. 10
1363:Simpson, R. J. (2010). "CTAB-PAGE".
1189:
1115:
40:discontinuous electrophoretic system
2537:Volume 869, 2012, p. 433–436,
1462:Volume 541, 2014, p. 151–159,
675:Two-dimensional gel electrophoresis
504:in aqueous solutions, tend to bind
243:) usually use the buffer substance
13:
3152:10.1111/j.1749-6632.1964.tb14207.x
1806:Volume 1721, 2018, p. 89–94,
1468:10.1016/B978-0-12-420119-4.00012-4
328:
14:
3271:
3237:
3052:. World Scientific. p. 142.
2509:. Academic Press. pp. 184–.
2296:Journal of Visualized Experiments
173:
601:post-translational modifications
83:Unfolding of a protein with heat
3123:
2899:
2848:
2624:
2605:
2215:
2161:
2118:
2075:
2040:
1996:
1935:
1860:
1480:Margolis J, Kenrick KG (1969).
1183:
709:
582:
413:
121:critical micellar concentration
75:Unfolding of a protein with SDS
1190:Berg, Jeremy M. (2015-04-08).
927:Trends in Biochemical Sciences
905:
893:(in German). No. 11. 2005
887:"Interview with Ulrich Lämmli"
879:
768:ammonium sulfate precipitation
320:In addition to the samples, a
249:continuous gel electrophoresis
1:
3244:Protocol for BisTris SDS-PAGE
2874:10.1016/S0021-9258(19)41496-8
2535:Methods in molecular biology.
2506:Guide to Protein Purification
2355:10.1016/s0076-6879(83)91020-0
1869:Biochimica et Biophysica Acta
1804:Methods in molecular biology.
1779:Methods in molecular biology.
1727:Journal of organic chemistry.
1290:10.1016/S0003-2697(02)00639-5
939:10.1016/s0968-0004(00)01679-0
810:
736:size exclusion chromatography
681:or BAC-PAGE with a SDS-PAGE.
310:tris(2-carboxyethyl)phosphine
284:of the protein by disrupting
66:
3048:Raja, Ghosh (11 June 2003).
2543:10.1007/978-1-61779-821-4_36
2183:10.1007/978-1-59745-281-6_21
2013:10.1016/0003-2697(79)90732-2
1921:10.1016/0003-2697(79)90581-5
1881:10.1016/0006-3002(63)91092-8
1838:10.1016/0003-2697(87)90587-2
1787:10.1007/978-1-4939-8793-1_12
1365:Cold Spring Harbor Protocols
1334:10.1016/0003-2697(85)90343-4
933:(12). Elsevier BV: 588–590.
732:tandem affinity purification
559:Molecular mass determination
532:
486:have differential levels of
322:molecular-weight size marker
161:
115:SDS tends to form spherical
7:
2425:Gilda JE, Gomes AV (2013).
2292:"Staining Proteins in Gels"
1812:10.1007/978-1-4939-7546-4_8
1237:Manning M, Colón W (2004).
1081:Colloid and Polymer Science
766:molecules, for example the
740:ion exchange chromatography
688:agarose gel electrophoresis
668:
474:
201:free radical polymerization
10:
3276:
3023:Desai, Mohamed A. (2000).
1454:J. L. Brunelle, R. Green:
777:
744:tangential flow filtration
698:can be detected via their
304:(DTT, 10–100 millimolar),
271:Disulfide reduction by DTT
208:and the radical initiator
3002:. John Wiley & Sons.
1775:Cationic Electrophoresis.
1702:10.3390/proteomes11010010
1093:10.1007/s00396-009-2059-3
374:gels differ by different
3187:Proc Natl Acad Sci U S A
2760:10.4103/1947-2714.100998
2443:10.1016/j.ab.2013.05.027
2404:10.1016/j.ab.2003.10.047
1949:Proc Natl Acad Sci U S A
1800:The No-Nonsens SDS-PAGE.
1798:L. Backman, K. Persson:
1554:10.1016/j.ab.2005.04.015
1048:10.1385/0-89603-062-8:41
985:Proc Natl Acad Sci U S A
799:in the working group of
788:Nobel Prize in Chemistry
719:methods with subsequent
622:, but less exact than a
2763:(inactive 2024-06-22).
2346:Enzyme Structure Part I
2139:10.1002/elps.1150130190
2096:10.1002/elps.1150090608
2061:10.1002/elps.1150080203
1826:Analytical Biochemistry
1598:10.1002/elps.1150120507
1542:Analytical Biochemistry
1412:Analytical Biochemistry
1322:Analytical Biochemistry
1278:Analytical Biochemistry
911:Neue Züricher Zeitung:
728:affinity chromatography
435:(highest sensitivity),
259:in the running buffer.
2855:O'Farrell, PH (1975).
2236:10.1002/pmic.201300089
1971:10.1073/pnas.76.9.4335
1649:10.1002/pmic.200600642
1460:Methods in enzymology.
1425:10.1006/abio.1996.0339
1006:10.1073/pnas.66.3.1002
690:can be used, e.g. the
677:sequentially combines
568:
542:
424:
350:
341:
308:(DTE, 10 millimolar),
300:(β-ME, 5% by volume),
272:
192:
183:
102:sodium dodecyl sulfate
84:
76:
56:sodium dodecyl sulfate
54:. The combined use of
28:
3208:10.1073/pnas.54.2.505
3092:10.1096/fj.08-0402ufm
3073:Pederson, T. (2007).
2825:10.2144/btn-2022-0003
659:Alpha-2-macroglobulin
566:
540:
421:
347:
336:
270:
189:
181:
129:quaternary structures
82:
74:
22:
16:Biochemical technique
1377:10.1101/pdb.prot5412
891:Neue Zürcher Zeitung
792:zone electrophoresis
679:isoelectric focusing
498:transmembrane domain
145:biological half-life
137:chemical equilibrium
3199:1965PNAS...54..505S
3144:1964NYASA.121..321O
2694:2015NatSR...513370G
2638:. 25 February 2021.
2531:Gel drying methods.
1962:1979PNAS...76.4335M
1735:10.1021/jo00008a014
1498:1969Natur.221.1056M
1371:(4): pdb.prot5412.
1169:10.1021/ja00486a062
997:1970PNAS...66.1002R
841:1970Natur.227..680L
653:in the urine, e.g.
620:ultracentrifugation
510:hydrophobic effects
496:, because of their
282:tertiary structures
210:ammonium persulfate
154:in a CTAB-PAGE, or
3246:at OpenWetWare.org
2969:10.1038/nmeth.2371
2926:10.1007/bf00281458
2682:Scientific Reports
772:polyethyleneglycol
569:
543:
529:can be performed.
425:
351:
342:
273:
263:Sample preparation
226:protein sequencing
193:
184:
85:
77:
29:
3079:The FASEB Journal
3059:978-1-78326-126-0
3034:978-1-59259-027-8
3009:978-1-118-00219-3
2906:Klose, J (1975).
2702:10.1038/srep13370
2662:978-94-011-1670-1
2598:978-3-540-32786-8
2571:978-1-119-95644-0
2516:978-0-08-092317-8
2489:978-0-08-095695-4
2276:978-3-642-18035-4
2192:978-1-58829-937-6
1760:978-0-8176-4412-3
1704:. PMID 36976889.
1506:10.1038/2211056a0
1255:10.1021/bi0491898
1163:(18): 5951–5952.
1133:978-0-470-08766-4
835:(5259): 680–685.
624:mass spectrometry
612:mass spectrometry
494:Membrane proteins
339:bromocresol green
325:pocket of a gel.
314:tributylphosphine
298:β-mercaptoethanol
290:disulfide bridges
218:radical scavenger
143:and an increased
44:Ulrich K. Laemmli
3267:
3231:
3230:
3220:
3210:
3178:
3172:
3171:
3127:
3121:
3120:
3094:
3070:
3064:
3063:
3045:
3039:
3038:
3020:
3014:
3013:
2995:
2989:
2988:
2952:
2946:
2945:
2911:
2903:
2897:
2896:
2886:
2876:
2852:
2846:
2845:
2827:
2803:
2797:
2796:
2790:
2782:
2772:
2762:
2738:
2732:
2731:
2721:
2673:
2667:
2666:
2646:
2640:
2639:
2628:
2622:
2621:
2618:www.ruf.rice.edu
2609:
2603:
2602:
2582:
2576:
2575:
2555:
2546:
2545:, PMID 22585507.
2527:
2521:
2520:
2500:
2494:
2493:
2474:
2465:
2464:
2454:
2422:
2416:
2415:
2383:
2377:
2376:
2340:
2334:
2333:
2323:
2287:
2281:
2280:
2262:
2256:
2255:
2219:
2213:
2212:
2176:
2165:
2159:
2158:
2122:
2116:
2115:
2079:
2073:
2072:
2044:
2038:
2037:
2031:
2023:
2000:
1994:
1993:
1983:
1973:
1956:(9): 4335–4339.
1939:
1933:
1932:
1904:
1893:
1892:
1864:
1858:
1857:
1821:
1815:
1814:, PMID 29423849.
1796:
1790:
1789:, PMID 30426413.
1771:
1765:
1764:
1744:
1738:
1719:
1713:
1686:
1677:
1676:
1632:
1626:
1625:
1580:
1574:
1573:
1537:
1526:
1525:
1492:(5185): 1056–7.
1477:
1471:
1470:, PMID 24674069.
1452:
1446:
1445:
1427:
1403:
1397:
1396:
1360:
1354:
1353:
1316:
1310:
1309:
1273:
1267:
1266:
1249:(35): 11248–54.
1234:
1228:
1227:
1221:
1213:
1187:
1181:
1180:
1152:
1146:
1145:
1119:
1113:
1112:
1087:(8): 1001–1004.
1076:
1070:
1069:
1035:
1029:
1028:
1018:
1008:
977:Tanford, Charles
972:
959:
958:
922:
916:
909:
903:
902:
900:
898:
883:
877:
876:
849:10.1038/227680a0
824:
805:bacteriophage T4
801:James E. Darnell
797:David F. Summers
786:was awarded the
637:and to evaluate
616:in-gel digestion
461:trichloroethanol
367:bromophenol blue
306:dithioerythritol
245:Bis-tris methane
168:western blotting
108:. SDS acts as a
23:Proteins of the
3275:
3274:
3270:
3269:
3268:
3266:
3265:
3264:
3260:Electrophoresis
3250:
3249:
3240:
3235:
3234:
3179:
3175:
3132:Ann NY Acad Sci
3128:
3124:
3071:
3067:
3060:
3046:
3042:
3035:
3021:
3017:
3010:
2996:
2992:
2953:
2949:
2904:
2900:
2867:(10): 4007–21.
2853:
2849:
2804:
2800:
2784:
2783:
2739:
2735:
2674:
2670:
2663:
2647:
2643:
2630:
2629:
2625:
2610:
2606:
2599:
2583:
2579:
2572:
2556:
2549:
2528:
2524:
2517:
2501:
2497:
2490:
2476:
2475:
2468:
2423:
2419:
2384:
2380:
2365:
2341:
2337:
2288:
2284:
2277:
2263:
2259:
2220:
2216:
2193:
2174:
2166:
2162:
2127:Electrophoresis
2123:
2119:
2084:Electrophoresis
2080:
2076:
2049:Electrophoresis
2045:
2041:
2025:
2024:
2001:
1997:
1940:
1936:
1905:
1896:
1865:
1861:
1822:
1818:
1797:
1793:
1772:
1768:
1761:
1745:
1741:
1720:
1716:
1687:
1680:
1633:
1629:
1586:Electrophoresis
1581:
1577:
1538:
1529:
1478:
1474:
1453:
1449:
1404:
1400:
1361:
1357:
1317:
1313:
1274:
1270:
1235:
1231:
1215:
1214:
1202:
1188:
1184:
1153:
1149:
1134:
1120:
1116:
1077:
1073:
1058:
1036:
1032:
973:
962:
923:
919:
910:
906:
896:
894:
885:
884:
880:
825:
818:
813:
780:
774:precipitation.
752:pull-down assay
748:ultrafiltration
717:chromatographic
712:
700:enzyme activity
671:
585:
561:
535:
477:
457:detection limit
441:Amido black 10B
433:silver staining
416:
331:
329:Electrophoresis
265:
176:
164:
158:in a BAC-PAGE.
89:electrophoresis
87:SDS-PAGE is an
69:
17:
12:
11:
5:
3273:
3263:
3262:
3248:
3247:
3239:
3238:External links
3236:
3233:
3232:
3173:
3138:(2): 321–349.
3122:
3085:(4): 949–953.
3065:
3058:
3040:
3033:
3015:
3008:
2990:
2963:(3): 211–220.
2947:
2898:
2847:
2798:
2753:(9): 429–434.
2733:
2668:
2661:
2641:
2623:
2604:
2597:
2577:
2570:
2547:
2522:
2515:
2495:
2488:
2466:
2417:
2378:
2363:
2335:
2282:
2275:
2257:
2230:(2–3): 162–8.
2214:
2191:
2160:
2133:(7): 429–439.
2117:
2090:(6): 288–291.
2074:
2039:
1995:
1934:
1894:
1859:
1832:(2): 368–379.
1816:
1791:
1766:
1759:
1739:
1714:
1678:
1643:(4): 524–527.
1627:
1592:(5): 352–366.
1575:
1548:(2): 237–245.
1527:
1472:
1447:
1418:(1): 126–133.
1398:
1355:
1328:(1): 170–176.
1311:
1268:
1229:
1200:
1182:
1147:
1132:
1114:
1071:
1056:
1030:
975:Reynolds, JA;
960:
917:
904:
878:
815:
814:
812:
809:
779:
776:
760:precipitations
726:, for example
724:quantification
711:
708:
670:
667:
651:serum proteins
584:
581:
560:
557:
534:
531:
527:gel extraction
523:
522:
518:
514:
491:
488:glycosylations
476:
473:
445:Fast green FCF
415:
412:
380:ionic strength
330:
327:
302:dithiothreitol
286:hydrogen bonds
264:
261:
237:gradient mixer
175:
174:Gel production
172:
163:
160:
68:
65:
60:polyacrylamide
15:
9:
6:
4:
3:
2:
3272:
3261:
3258:
3257:
3255:
3245:
3242:
3241:
3228:
3224:
3219:
3214:
3209:
3204:
3200:
3196:
3193:(2): 505–13.
3192:
3188:
3184:
3177:
3169:
3165:
3161:
3157:
3153:
3149:
3145:
3141:
3137:
3133:
3126:
3118:
3114:
3110:
3106:
3102:
3098:
3093:
3088:
3084:
3080:
3076:
3069:
3061:
3055:
3051:
3044:
3036:
3030:
3026:
3019:
3011:
3005:
3001:
2994:
2986:
2982:
2978:
2974:
2970:
2966:
2962:
2958:
2951:
2943:
2939:
2935:
2931:
2927:
2923:
2920:(3): 231–43.
2919:
2915:
2910:
2902:
2894:
2890:
2885:
2880:
2875:
2870:
2866:
2862:
2861:J. Biol. Chem
2858:
2851:
2843:
2839:
2835:
2831:
2826:
2821:
2817:
2813:
2812:BioTechniques
2809:
2802:
2794:
2788:
2780:
2776:
2771:
2766:
2761:
2756:
2752:
2748:
2744:
2737:
2729:
2725:
2720:
2715:
2711:
2707:
2703:
2699:
2695:
2691:
2687:
2683:
2679:
2672:
2664:
2658:
2654:
2653:
2645:
2637:
2636:Peak Proteins
2633:
2627:
2619:
2615:
2608:
2600:
2594:
2590:
2589:
2581:
2573:
2567:
2563:
2562:
2554:
2552:
2544:
2540:
2536:
2532:
2526:
2518:
2512:
2508:
2507:
2499:
2491:
2485:
2481:
2480:
2473:
2471:
2462:
2458:
2453:
2448:
2444:
2440:
2436:
2432:
2428:
2421:
2413:
2409:
2405:
2401:
2397:
2393:
2389:
2382:
2374:
2370:
2366:
2364:9780121819910
2360:
2356:
2352:
2348:
2347:
2339:
2331:
2327:
2322:
2317:
2313:
2309:
2305:
2301:
2297:
2293:
2286:
2278:
2272:
2268:
2261:
2253:
2249:
2245:
2241:
2237:
2233:
2229:
2225:
2218:
2210:
2206:
2202:
2198:
2194:
2188:
2184:
2180:
2173:
2172:
2164:
2156:
2152:
2148:
2144:
2140:
2136:
2132:
2128:
2121:
2113:
2109:
2105:
2101:
2097:
2093:
2089:
2085:
2078:
2070:
2066:
2062:
2058:
2054:
2050:
2043:
2035:
2029:
2022:
2018:
2014:
2010:
2006:
1999:
1991:
1987:
1982:
1977:
1972:
1967:
1963:
1959:
1955:
1951:
1950:
1945:
1938:
1930:
1926:
1922:
1918:
1915:(2): 263–78.
1914:
1910:
1903:
1901:
1899:
1890:
1886:
1882:
1878:
1874:
1870:
1863:
1855:
1851:
1847:
1843:
1839:
1835:
1831:
1827:
1820:
1813:
1809:
1805:
1801:
1795:
1788:
1784:
1780:
1776:
1770:
1762:
1756:
1752:
1751:
1743:
1736:
1732:
1728:
1724:
1718:
1711:
1707:
1703:
1699:
1695:
1691:
1685:
1683:
1674:
1670:
1666:
1662:
1658:
1654:
1650:
1646:
1642:
1638:
1631:
1623:
1619:
1615:
1611:
1607:
1603:
1599:
1595:
1591:
1587:
1579:
1571:
1567:
1563:
1559:
1555:
1551:
1547:
1543:
1536:
1534:
1532:
1523:
1519:
1515:
1511:
1507:
1503:
1499:
1495:
1491:
1487:
1483:
1476:
1469:
1465:
1461:
1457:
1451:
1443:
1439:
1435:
1431:
1426:
1421:
1417:
1413:
1409:
1402:
1394:
1390:
1386:
1382:
1378:
1374:
1370:
1366:
1359:
1351:
1347:
1343:
1339:
1335:
1331:
1327:
1323:
1315:
1307:
1303:
1299:
1295:
1291:
1287:
1283:
1279:
1272:
1264:
1260:
1256:
1252:
1248:
1244:
1240:
1233:
1225:
1219:
1211:
1207:
1203:
1201:9781464126109
1197:
1193:
1186:
1178:
1174:
1170:
1166:
1162:
1158:
1151:
1143:
1139:
1135:
1129:
1125:
1118:
1110:
1106:
1102:
1098:
1094:
1090:
1086:
1082:
1075:
1067:
1063:
1059:
1057:0-89603-062-8
1053:
1049:
1045:
1041:
1034:
1026:
1022:
1017:
1012:
1007:
1002:
998:
994:
991:(3): 1002–7.
990:
986:
982:
978:
971:
969:
967:
965:
956:
952:
948:
944:
940:
936:
932:
928:
921:
914:
908:
892:
888:
882:
874:
870:
866:
862:
858:
854:
850:
846:
842:
838:
834:
830:
823:
821:
816:
808:
806:
802:
798:
793:
789:
785:
784:Arne Tiselius
775:
773:
769:
765:
761:
757:
753:
749:
745:
741:
737:
733:
729:
725:
722:
718:
707:
705:
701:
697:
693:
689:
684:
680:
676:
666:
664:
660:
656:
652:
648:
644:
640:
636:
631:
629:
625:
621:
617:
613:
608:
606:
602:
598:
593:
591:
580:
578:
573:
565:
556:
553:
549:
539:
530:
528:
519:
515:
511:
507:
503:
499:
495:
492:
489:
485:
484:Glycoproteins
482:
481:
480:
472:
469:
464:
462:
458:
454:
450:
446:
442:
438:
434:
430:
420:
411:
409:
404:
400:
397:
393:
389:
385:
381:
377:
371:
368:
362:
360:
356:
346:
340:
335:
326:
323:
318:
315:
311:
307:
303:
299:
295:
291:
287:
283:
279:
269:
260:
258:
254:
250:
246:
242:
241:pre-cast gels
238:
233:
232:in proteins.
231:
227:
223:
219:
215:
211:
207:
202:
198:
188:
180:
171:
169:
159:
157:
153:
148:
146:
142:
138:
134:
133:cross-linking
130:
126:
122:
118:
113:
111:
107:
103:
99:
95:
90:
81:
73:
64:
61:
57:
53:
49:
45:
42:developed by
41:
37:
33:
26:
21:
3190:
3186:
3176:
3135:
3131:
3125:
3082:
3078:
3068:
3049:
3043:
3024:
3018:
2999:
2993:
2960:
2956:
2950:
2917:
2914:Humangenetik
2913:
2901:
2864:
2860:
2850:
2818:(1): 58–69.
2815:
2811:
2801:
2787:cite journal
2750:
2746:
2736:
2688:(1): 13370.
2685:
2681:
2671:
2651:
2644:
2635:
2626:
2617:
2607:
2587:
2580:
2560:
2534:
2530:
2525:
2505:
2498:
2478:
2437:(2): 186–8.
2434:
2431:Anal Biochem
2430:
2420:
2398:(1): 13–20.
2395:
2392:Anal Biochem
2391:
2381:
2345:
2338:
2295:
2285:
2269:. Springer.
2266:
2260:
2227:
2223:
2217:
2170:
2163:
2130:
2126:
2120:
2087:
2083:
2077:
2052:
2048:
2042:
2005:Anal Biochem
2004:
1998:
1953:
1947:
1937:
1912:
1909:Anal Biochem
1908:
1872:
1868:
1862:
1829:
1825:
1819:
1803:
1799:
1794:
1778:
1774:
1773:E. Buxbaum:
1769:
1749:
1742:
1726:
1722:
1717:
1693:
1689:
1640:
1636:
1630:
1589:
1585:
1578:
1545:
1541:
1489:
1485:
1475:
1459:
1455:
1450:
1415:
1411:
1401:
1368:
1364:
1358:
1325:
1321:
1314:
1284:(1): 70–76.
1281:
1277:
1271:
1246:
1243:Biochemistry
1242:
1232:
1192:Biochemistry
1191:
1185:
1160:
1156:
1150:
1123:
1117:
1084:
1080:
1074:
1039:
1033:
988:
984:
930:
926:
920:
907:
895:. Retrieved
890:
881:
832:
828:
781:
713:
710:Alternatives
672:
632:
628:DNA sequence
609:
604:
597:western blot
594:
586:
583:Applications
574:
570:
544:
524:
478:
465:
453:Western Blot
449:epicocconone
426:
414:Gel staining
403:zwitterionic
372:
363:
352:
319:
274:
240:
234:
222:filter paper
194:
165:
149:
114:
86:
35:
31:
30:
2957:Nat Methods
2304:10.3791/760
1875:: 377–391.
764:kosmotropic
756:extractions
721:photometric
683:Native PAGE
647:blood serum
639:proteinuria
590:scalability
235:By using a
106:amino acids
96:. Although
25:erythrocyte
2224:Proteomics
1694:Proteomes.
1637:Proteomics
811:References
704:zymography
643:antibodies
605:band shift
548:cellophane
502:solubility
443:staining,
439:staining,
437:stains all
423:separated.
110:surfactant
67:Properties
3101:0892-6638
2842:250175915
2710:2045-2322
2312:1940-087X
2252:206368546
2055:: 93–99.
1846:0003-2697
1657:1615-9853
1606:0173-0835
1562:0003-2697
1434:0003-2697
1385:1559-6095
1342:0003-2697
1298:0003-2697
1218:cite book
1210:913469736
1177:0002-7863
1142:420027217
1101:0303-402X
947:0968-0004
857:0028-0836
782:In 1948,
730:(or even
533:Archiving
468:Coomassie
429:Coomassie
384:pH values
278:secondary
230:cysteines
197:diffusion
191:unstained
162:Procedure
141:proteases
98:tube gels
3254:Category
3168:28591995
3160:14240533
3117:33466516
3109:18378803
2977:23443633
2942:30981877
2834:35775367
2779:23050259
2728:26311515
2461:23747530
2412:14769330
2330:19066521
2244:24339236
2209:52820065
2201:19381593
2155:43084621
2112:33007991
2069:84471792
2028:citation
1889:18421828
1710:10059017
1673:25822873
1665:17309097
1622:40101706
1570:15935323
1393:20360366
1306:12633604
1263:15366934
1109:97367384
1066:20512673
1040:Proteins
979:(1970).
955:11116182
897:March 4,
770:and the
669:Variants
635:HIV test
577:histones
552:glycerol
517:covered.
475:Analysis
396:chloride
296:such as
214:meniscus
117:micelles
94:slab gel
48:proteins
32:SDS-PAGE
3227:4285933
3195:Bibcode
3140:Bibcode
2985:5314901
2934:1093965
2884:2874754
2770:3456489
2719:4550835
2690:Bibcode
2452:3809032
2373:6190068
2321:3253607
2147:1425556
2104:2466660
1958:Bibcode
1854:2449095
1614:1718736
1522:4197850
1514:5774398
1494:Bibcode
1442:8811889
1350:4003759
1025:5269225
993:Bibcode
873:3105149
865:5432063
837:Bibcode
778:History
696:enzymes
694:. Some
692:SDD-AGE
655:Albumin
408:Tricine
392:glycine
355:voltage
125:monomer
38:) is a
3225:
3218:219696
3215:
3166:
3158:
3115:
3107:
3099:
3056:
3031:
3006:
2983:
2975:
2940:
2932:
2893:236308
2891:
2881:
2840:
2832:
2777:
2767:
2726:
2716:
2708:
2659:
2595:
2568:
2513:
2486:
2459:
2449:
2410:
2371:
2361:
2328:
2318:
2310:
2298:(17).
2273:
2250:
2242:
2207:
2199:
2189:
2153:
2145:
2110:
2102:
2067:
2019:
1988:
1981:411569
1978:
1927:
1887:
1852:
1844:
1757:
1708:
1671:
1663:
1655:
1620:
1612:
1604:
1568:
1560:
1520:
1512:
1486:Nature
1440:
1432:
1391:
1383:
1348:
1340:
1304:
1296:
1261:
1208:
1198:
1175:
1140:
1130:
1107:
1099:
1064:
1054:
1023:
1016:283150
1013:
953:
945:
871:
863:
855:
829:Nature
762:using
746:or an
506:lipids
399:buffer
294:thiols
156:16-BAC
3164:S2CID
3113:S2CID
2981:S2CID
2938:S2CID
2838:S2CID
2248:S2CID
2205:S2CID
2175:(PDF)
2151:S2CID
2108:S2CID
2065:S2CID
2021:94518
1990:92027
1929:89822
1669:S2CID
1618:S2CID
1518:S2CID
1105:S2CID
869:S2CID
359:anode
206:TEMED
3223:PMID
3156:PMID
3105:PMID
3097:ISSN
3054:ISBN
3029:ISBN
3004:ISBN
2973:PMID
2930:PMID
2889:PMID
2830:PMID
2793:link
2775:PMID
2724:PMID
2706:ISSN
2657:ISBN
2593:ISBN
2566:ISBN
2533:In:
2511:ISBN
2484:ISBN
2457:PMID
2408:PMID
2369:PMID
2359:ISBN
2326:PMID
2308:ISSN
2271:ISBN
2240:PMID
2197:PMID
2187:ISBN
2143:PMID
2100:PMID
2034:link
2017:PMID
1986:PMID
1925:PMID
1885:PMID
1850:PMID
1842:ISSN
1802:In:
1777:In:
1755:ISBN
1725:In:
1692:In:
1661:PMID
1653:ISSN
1610:PMID
1602:ISSN
1566:PMID
1558:ISSN
1510:PMID
1458:In:
1438:PMID
1430:ISSN
1389:PMID
1381:ISSN
1369:2010
1346:PMID
1338:ISSN
1302:PMID
1294:ISSN
1259:PMID
1224:link
1206:OCLC
1196:ISBN
1173:ISSN
1138:OCLC
1128:ISBN
1097:ISSN
1062:PMID
1052:ISBN
1021:PMID
951:PMID
943:ISSN
899:2012
861:PMID
853:ISSN
758:and
661:and
388:Tris
382:and
376:pore
349:SDS.
280:and
257:MOPS
152:CTAB
3213:PMC
3203:doi
3148:doi
3136:121
3087:doi
2965:doi
2922:doi
2879:PMC
2869:doi
2865:250
2820:doi
2765:PMC
2755:doi
2714:PMC
2698:doi
2539:doi
2447:PMC
2439:doi
2435:440
2400:doi
2396:326
2351:doi
2316:PMC
2300:doi
2232:doi
2179:doi
2135:doi
2092:doi
2057:doi
2009:doi
1976:PMC
1966:doi
1917:doi
1877:doi
1834:doi
1830:166
1808:doi
1783:doi
1731:doi
1706:PMC
1698:doi
1645:doi
1594:doi
1550:doi
1546:342
1502:doi
1490:221
1464:doi
1420:doi
1416:240
1373:doi
1330:doi
1326:145
1286:doi
1282:314
1251:doi
1165:doi
1161:100
1089:doi
1085:287
1044:doi
1011:PMC
1001:doi
935:doi
845:doi
833:227
734:),
702:by
663:IgG
610:In
466:In
312:or
255:or
253:MES
52:kDa
3256::
3221:.
3211:.
3201:.
3191:54
3189:.
3185:.
3162:.
3154:.
3146:.
3134:.
3111:.
3103:.
3095:.
3083:22
3081:.
3077:.
2979:.
2971:.
2961:10
2959:.
2936:.
2928:.
2918:26
2916:.
2912:.
2887:.
2877:.
2863:.
2859:.
2836:.
2828:.
2816:73
2814:.
2810:.
2789:}}
2785:{{
2773:.
2749:.
2745:.
2722:.
2712:.
2704:.
2696:.
2684:.
2680:.
2634:.
2616:.
2550:^
2469:^
2455:.
2445:.
2433:.
2429:.
2406:.
2394:.
2390:.
2367:.
2357:.
2324:.
2314:.
2306:.
2294:.
2246:.
2238:.
2228:14
2226:.
2203:.
2195:.
2185:.
2149:.
2141:.
2131:13
2129:.
2106:.
2098:.
2086:.
2063:.
2051:.
2030:}}
2026:{{
2015:,
1984:.
1974:.
1964:.
1954:76
1952:.
1946:.
1923:.
1913:96
1911:.
1897:^
1883:.
1873:71
1871:.
1848:.
1840:.
1828:.
1681:^
1667:.
1659:.
1651:.
1639:.
1616:.
1608:.
1600:.
1590:12
1588:.
1564:.
1556:.
1544:.
1530:^
1516:.
1508:.
1500:.
1488:.
1484:.
1436:.
1428:.
1414:.
1410:.
1387:.
1379:.
1367:.
1344:.
1336:.
1324:.
1300:.
1292:.
1280:.
1257:.
1247:43
1245:.
1241:.
1220:}}
1216:{{
1204:.
1171:.
1159:.
1136:.
1103:.
1095:.
1083:.
1060:.
1050:.
1019:.
1009:.
999:.
989:66
987:.
983:.
963:^
949:.
941:.
931:25
929:.
889:.
867:.
859:.
851:.
843:.
831:.
819:^
807:.
738:,
706:.
665:.
657:,
630:.
147:.
3229:.
3205::
3197::
3170:.
3150::
3142::
3119:.
3089::
3062:.
3037:.
3012:.
2987:.
2967::
2944:.
2924::
2895:.
2871::
2844:.
2822::
2795:)
2781:.
2757::
2751:4
2730:.
2700::
2692::
2686:5
2665:.
2620:.
2601:.
2574:.
2541::
2519:.
2492:.
2463:.
2441::
2414:.
2402::
2375:.
2353::
2332:.
2302::
2279:.
2254:.
2234::
2211:.
2181::
2157:.
2137::
2114:.
2094::
2088:9
2071:.
2059::
2053:8
2036:)
2011::
1992:.
1968::
1960::
1931:.
1919::
1891:.
1879::
1856:.
1836::
1810::
1785::
1763:.
1737:.
1733::
1712:.
1700::
1675:.
1647::
1641:7
1624:.
1596::
1572:.
1552::
1524:.
1504::
1496::
1466::
1444:.
1422::
1395:.
1375::
1352:.
1332::
1308:.
1288::
1265:.
1253::
1226:)
1212:.
1179:.
1167::
1144:.
1111:.
1091::
1068:.
1046::
1027:.
1003::
995::
957:.
937::
901:.
875:.
847::
839::
394:-
390:-
34:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.