397:) of two straight lines traced through plots of the measured property versus the surfactant concentration. This visual data analysis method is highly subjective and can lead to very different CMC values depending on the type of representation, the quality of the data and the chosen interval around the CMC. A preferred method is the fit of the experimental data with a model of the measured property. Fit functions for properties such as electrical conductivity, surface tension, NMR chemical shifts, absorption, self-diffusion coefficients, fluorescence intensity and mean translational diffusion coefficient of fluorescent dyes in surfactant solutions have been presented. These fit functions are based on a model for the concentrations of monomeric and micellised surfactants in solution, which establishes a well-defined analytical definition of the CMC, independent from the technique.
426:
211:
448:(EOR) application. Below the CMC point, interfacial tension between oil and water phase is no longer effectively reduced. If the concentration of the surfactant is kept a little above the CMC, the additional amount covers the dissolution with existing brine in the reservoir. It is desired that the surfactant will work at the lowest interfacial tension (IFT).
417:
When the interfacial areas are large, the amount of surfactant at the interface cannot be neglected. If, for example, air bubbles are introduced into a solution of a surfactant above CMC, these bubbles, as they rise to the surface, remove surfactants from the bulk to the top of the solution creating
404:
is important because surfactants partition between the bulk and interface and CMC is independent of interface and is therefore a characteristic of the surfactant molecule. In most situations, such as surface tension measurements or conductivity measurements, the amount of surfactant at the interface
61:
changes strongly with the concentration of the surfactant. After reaching the CMC, the surface tension remains relatively constant or changes with a lower slope. The value of the CMC for a given dispersant in a given medium depends on temperature, pressure, and (sometimes strongly) on the presence
234:
Subsequently, when the surface coverage by the surfactants increases, the surface free energy (surface tension) decreases and the surfactants start aggregating into micelles, thus again decreasing the system's free energy by decreasing the contact area of hydrophobic parts of the surfactant with
214:
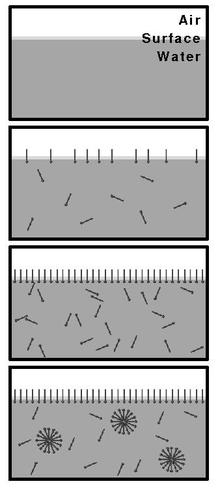
Top to Bottom: Increasing concentration of surfactant in water first leads to the formation of a layer on the surface. After reaching the CMC micelles begin forming. Notice that the existence of micelles does not preclude the existence of individual surfactant molecules in
418:
a foam column and thus reducing the concentration in bulk to below CMC. This is one of the easiest methods to remove surfactants from effluents (see foam flotation). Thus in foams with sufficient interfacial area are devoid of micelles. Similar reasoning holds for
405:
is negligible compared to that in the bulk and CMC can be approximated by the total concentration. In practice, CMC data is usually collected using laboratory instruments which allow the process to be partially automated, for instance by using specialised
734:
Lucas Piñeiro, Sonia Freire, Jorge
Bordello, Mercedes Novo, and Wajih Al-Soufi, Dye Exchange in Micellar Solutions. Quantitative Analysis of Bulk and Single Molecule Fluorescence Titrations. Soft Matter, 2013,9, 10779-10790, DOI:
437:. One initially starts off with concentrations greater than CMC in water and on adding fabric with large interfacial area, the surfactant concentration drops below CMC and no micelles remain at equilibrium. Therefore, the
668:
Mukerjee, P.; Mysels, K. J. In
Critical Micelle Concentrations of Aqueous Surfactant Systems; NIST National Institute of Standards and Technology: Washington D.C. USA, 1971; Vol. NSRDS-NBS 36
753:
294:
264:
574:
491:
17:
680:"A model for monomer and micellar concentrations in surfactant solutions: Application to conductivity, NMR, diffusion, and surface tension data"
441:
plays a minor role in detergents. Removal of oily soil occurs by modification of the contact angles and release of oil in the form of emulsion.
780:
745:
638:
191:
550:
235:
water. Upon reaching CMC, any further addition of surfactants will just increase the number of micelles (in the ideal case).
429:
CMC is most typically measured by plotting surface tension versus surfactant concentration in an automated measurement.
156:
591:
219:
Upon introducing surfactants (or any surface active materials) into a system, they will initially partition into the
425:
386:, then the CMC is not related to the method of measurement. On the other hand, when the degree of aggregation is
805:
406:
238:
According to one well-known definition, CMC is the total concentration of surfactants under the conditions:
825:
777:"A Critical Review on Microbial Enhanced Oil Recovery Using Artificial Sandstone Core: Mathematical Model"
679:
521:
Dominguez, Ana; Fernandez, Aurora; Gonzalez, Noemi; Iglesias, Emilia; Montenegro, Luis (October 1997).
121:
776:
659:
Phillips J. The energetics of micelle formation. Transactions of the
Faraday Society 1955;51:561-9
400:
The CMC is the concentration of surfactants in the bulk at which micelles start forming. The word
393:
The common procedure to determine the CMC from experimental data is to look for the intersection (
617:"Surfactant-Polymer Coreflood Simulation and Uncertainty Analysis Derived from Laboratory Study"
77:
in water (without other additives or salts) at 25 °C, atmospheric pressure, is 8x10 mol/L.
810:
371:
108:
74:
616:
444:
In petroleum industry, CMC is considered prior to injecting surfactant in reservoir regarding
445:
220:
279:
249:
633:
8:
523:"Determination of Critical Micelle Concentration of Some Surfactants by Three Techniques"
31:
597:
67:
717:
709:
601:
587:
542:
57:
The CMC is an important characteristic of a surfactant. Before reaching the CMC, the
35:
501:
227:
lowering the energy of the interface (calculated as area times surface tension), and
699:
691:
628:
579:
534:
505:
496:
394:
583:
522:
615:
Hakiki, Farizal; Maharsi, Dara Ayuda; Marhaendrajana, Taufan (31 December 2015).
467:
379:
58:
695:
438:
819:
713:
546:
500:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
375:
509:
390:, then CMC is related to both the method of measurement and the dispersion.
54:
form and all additional surfactants added to the system will form micelles.
721:
387:
383:
230:
removing the hydrophobic parts of the surfactant from contact with water.
63:
362:
The CMC generally depends on the method of measuring the samples, since
704:
538:
472:
47:
457:
434:
419:
520:
678:
Al-Soufi, Wajih; Piñeiro, Lucas; Novo, Mercedes (15 March 2012).
462:
51:
811:
CMCs and molecular weights of several detergents on OpenWetWare
746:"Concentration-model for surfactants near the cmc | QBFE -USC"
614:
62:
and concentration of other surface active substances and
210:
27:
Concentration of surfactants above which micelles form
353:= represents the number of detergent ions per micelle
282:
252:
677:
288:
258:
621:Journal of Engineering and Technological Sciences
370:depend on the properties of the solution such as
817:
575:Ullmann's Encyclopedia of Industrial Chemistry
412:
703:
632:
572:Holmberg, Krister (2019). "Surfactants".
684:Journal of Colloid and Interface Science
571:
424:
209:
14:
818:
774:
578:. Weinheim: Wiley-VCH. pp. 1–56.
223:, reducing the system free energy by:
192:Pentaethylene glycol monododecyl ether
634:10.5614/j.eng.technol.sci.2015.47.6.9
46:) is defined as the concentration of
783:from the original on 5 November 2022
382:. When the degree of aggregation is
180:Penta(ethyleneglycol)monodecyl ether
169:Penta(ethyleneglycol)monooctyl ether
641:from the original on 20 August 2016
24:
553:from the original on 13 March 2023
497:Compendium of Chemical Terminology
157:Hexadecyltrimethylammonium bromide
73:For example, the value of CMC for
25:
837:
799:
756:from the original on 4 April 2017
145:Dodecyltrimethylammonium bromide
768:
18:Critical micellar concentration
738:
728:
671:
662:
653:
608:
565:
514:
502:critical micelle concentration
485:
433:The other situation arises in
357:
205:
134:Decyltrimethylammonium bromide
40:critical micelle concentration
13:
1:
584:10.1002/14356007.a25_747.pub2
527:Journal of Chemical Education
478:
327:are proportionality constants
81:CMCs for common surfactants
68:critical micelle temperature
7:
451:
66:. Micelles only form above
10:
842:
696:10.1016/j.jcis.2011.12.037
806:Theory of CMC measurement
122:Sodium tetradecyl sulfate
775:Hakiki, Farizal (2014).
413:Practical considerations
510:10.1351/goldbook.C01395
430:
290:
260:
216:
109:Sodium dodecyl sulfate
75:sodium dodecyl sulfate
446:enhanced oil recovery
428:
291:
289:{\displaystyle \phi }
261:
259:{\displaystyle \phi }
213:
378:characteristics, or
280:
250:
164:cationic surfactant
151:cationic surfactant
140:cationic surfactant
97:Sodium octyl sulfate
826:Colloidal chemistry
199:neutral surfactant
186:neutral surfactant
175:neutral surfactant
129:anionic surfactant
116:anionic surfactant
103:anionic surfactant
82:
735:10.1039/c3sm52092g
539:10.1021/ed074p1227
431:
286:
256:
217:
80:
533:(10): 1227–1231.
305:; i.e., in words
203:
202:
36:surface chemistry
16:(Redirected from
833:
793:
792:
790:
788:
772:
766:
765:
763:
761:
742:
736:
732:
726:
725:
707:
675:
669:
666:
660:
657:
651:
650:
648:
646:
636:
612:
606:
605:
569:
563:
562:
560:
558:
518:
512:
489:
395:inflection point
295:
293:
292:
287:
265:
263:
262:
257:
83:
79:
21:
841:
840:
836:
835:
834:
832:
831:
830:
816:
815:
802:
797:
796:
786:
784:
773:
769:
759:
757:
744:
743:
739:
733:
729:
676:
672:
667:
663:
658:
654:
644:
642:
613:
609:
594:
570:
566:
556:
554:
519:
515:
490:
486:
481:
468:Surface tension
454:
415:
380:surface tension
360:
345:
339:
334:
318:
311:
281:
278:
277:
271:
251:
248:
247:
208:
59:surface tension
28:
23:
22:
15:
12:
11:
5:
839:
829:
828:
814:
813:
808:
801:
800:External links
798:
795:
794:
767:
737:
727:
690:(1): 102–110.
670:
661:
652:
627:(6): 706–725.
607:
592:
564:
513:
483:
482:
480:
477:
476:
475:
470:
465:
460:
453:
450:
439:solubilization
414:
411:
359:
356:
355:
354:
343:
337:
332:
328:
316:
309:
285:
274:
269:
255:
232:
231:
228:
207:
204:
201:
200:
197:
194:
188:
187:
184:
181:
177:
176:
173:
170:
166:
165:
162:
159:
153:
152:
149:
146:
142:
141:
138:
135:
131:
130:
127:
124:
118:
117:
114:
111:
105:
104:
101:
98:
94:
93:
90:
89:CMC (molarity)
87:
26:
9:
6:
4:
3:
2:
838:
827:
824:
823:
821:
812:
809:
807:
804:
803:
782:
778:
771:
755:
751:
747:
741:
731:
723:
719:
715:
711:
706:
701:
697:
693:
689:
685:
681:
674:
665:
656:
640:
635:
630:
626:
622:
618:
611:
603:
599:
595:
593:9783527306732
589:
585:
581:
577:
576:
568:
552:
548:
544:
540:
536:
532:
528:
524:
517:
511:
507:
503:
499:
498:
493:
488:
484:
474:
471:
469:
466:
464:
461:
459:
456:
455:
449:
447:
442:
440:
436:
427:
423:
421:
410:
408:
403:
398:
396:
391:
389:
385:
381:
377:
376:photochemical
373:
369:
365:
352:
348:
342:
336:
329:
326:
322:
315:
308:
304:
300:
296:
283:
275:
272:
253:
245:
241:
240:
239:
236:
229:
226:
225:
224:
222:
212:
198:
195:
193:
190:
189:
185:
182:
179:
178:
174:
171:
168:
167:
163:
160:
158:
155:
154:
150:
147:
144:
143:
139:
136:
133:
132:
128:
125:
123:
120:
119:
115:
112:
110:
107:
106:
102:
99:
96:
95:
91:
88:
85:
84:
78:
76:
71:
69:
65:
60:
55:
53:
49:
45:
41:
37:
33:
19:
785:. Retrieved
770:
758:. Retrieved
749:
740:
730:
687:
683:
673:
664:
655:
643:. Retrieved
624:
620:
610:
573:
567:
555:. Retrieved
530:
526:
516:
495:
487:
443:
432:
416:
407:tensiometers
401:
399:
392:
388:polydisperse
384:monodisperse
367:
363:
361:
350:
346:
340:
330:
324:
320:
313:
306:
302:
298:
276:
267:
243:
237:
233:
218:
72:
64:electrolytes
56:
50:above which
43:
39:
29:
760:18 December
705:10347/17083
372:conductance
358:Measurement
206:Description
48:surfactants
787:5 November
479:References
473:Surfactant
435:detergents
86:Surfactant
714:0021-9797
602:242339510
547:0021-9584
458:Detergent
420:emulsions
284:ϕ
254:ϕ
246:= CMC, (d
221:interface
215:solution.
92:Category
32:colloidal
820:Category
781:Archived
754:Archived
722:22265231
639:Archived
551:Archived
452:See also
196:0.000065
52:micelles
463:Micelle
319:= and
161:0.00092
750:usc.es
720:
712:
645:1 July
600:
590:
557:2 June
545:
349:i.e.,
183:0.0009
172:0.0009
126:0.0021
113:0.0083
38:, the
598:S2CID
492:IUPAC
312:= ,
273:) = 0
148:0.016
137:0.065
789:2022
762:2023
718:PMID
710:ISSN
647:2016
588:ISBN
559:2023
543:ISSN
402:bulk
366:and
341:+ NC
323:and
100:0.13
34:and
700:hdl
692:doi
688:370
629:doi
580:doi
535:doi
506:doi
504:".
335:= C
242:if
44:CMC
30:In
822::
779:.
752:.
748:.
716:.
708:.
698:.
686:.
682:.
637:.
625:47
623:.
619:.
596:.
586:.
549:.
541:.
531:74
529:.
525:.
494:,
422:.
409:.
374:,
301:+
297:=
266:/d
70:.
791:.
764:.
724:.
702::
694::
649:.
631::
604:.
582::
561:.
537::
508::
368:B
364:A
351:N
347:;
344:m
338:s
333:t
331:C
325:B
321:A
317:m
314:C
310:s
307:C
303:B
299:A
270:t
268:C
244:C
42:(
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.