278:
782:
2462:
38:
768:
457:
610:
1183:
reforming does not eliminate carbon dioxide release into the atmosphere but reduces the carbon dioxide emissions and nearly eliminates carbon monoxide emissions as compared to the burning of conventional fuels due to increased efficiency and fuel cell characteristics. However, by turning the release
1036:
1178:
The reformer– the fuel-cell system is still being researched but in the near term, systems would continue to run on existing fuels, such as natural gas or gasoline or diesel. However, there is an active debate about whether using these fuels to make hydrogen is beneficial while global warming
1071:
The capital cost of steam reforming plants is considered prohibitive for small to medium size applications. The costs for these elaborate facilities do not scale down well. Conventional steam reforming plants operate at pressures between 200 and 600 psi (14–40 bar) with outlet temperatures in the
855:
produces 9–10 million tons of hydrogen per year, mostly with steam reforming of natural gas. The worldwide ammonia production, using hydrogen derived from steam reforming, was 144 million tonnes in 2018. The energy consumption has been reduced from 100 GJ/tonne of ammonia in 1920 to 27 GJ by 2019.
1228:
would be another cause of catalyst deactivation during steam reforming. High reaction temperatures, low steam-to-carbon ratio (S/C), and the complex nature of sulfur-containing commercial hydrocarbon fuels make coking especially favorable. Olefins, typically ethylene, and aromatics are well-known
1062:
Partial oxidation (POX) occurs when a sub-stoichiometric fuel-air mixture is partially combusted in a reformer creating hydrogen-rich syngas. POX is typically much faster than steam reforming and requires a smaller reactor vessel. POX produces less hydrogen per unit of the input fuel than steam
772:
As these reactions by themselves are highly endothermic (apart from WGSR, which is mildly exothermic), a large amount of heat needs to be added to the reactor to keep a constant temperature. Optimal SMR reactor operating conditions lie within a temperature range of 800 °C to 900 °C at
1237:
of reforming reactants. Meanwhile, the adsorbed sulfur species increases the catalyst acidity, and hence indirectly promotes coking. Precious metal catalysts such as Rh and Pt have lower tendencies to form bulk sulfides than other metal catalysts such as Ni. Rh and Pt are less prone to sulfur
1199:
depends on the scale at which it is done, the capital cost of the reformer, and the efficiency of the unit, so that whilst it may cost only a few dollars per kilogram of hydrogen at an industrial scale, it could be more expensive at the smaller scale needed for fuel cells.
1087:(VOCs) are known problems in the offshore industry and in the on-shore oil and gas industry, since both release greenhouse gases into the atmosphere. Reforming for combustion engines utilizes steam reforming technology for converting waste gases into a source of energy.
801:, providing the necessary energy to keep the reactor at a constant temperature during operation. Furnace designs vary, depending on the burner configuration they are typically categorized into: top-fired, bottom-fired, and side-fired. A notable design is the
619:
1044::CO ratio can be varied, which can be useful for producing specialty products. Due to the exothermic nature of some of the additional reactions occurring within ATR, the process can essentially be performed at a net enthalpy of zero (Δ
322:
470:
902:
1229:
carbon-precursors, hence their formation must be reduced during steam reforming. Additionally, catalysts with lower acidity were reported to be less prone to coking by suppressing dehydrogenation reactions. H
2108:
2249:
Zheng, Qinghe; Janke, Christiane; Farrauto, Robert (2014). "Steam reforming of sulfur-containing dodecane on a Rh–Pt catalyst: Influence of process parameters on catalyst stability and coke structure".
195:
1233:
S, the main product in the reforming of organic sulfur, can bind to all transition metal catalysts to form metal–sulfur bonds and subsequently reduce catalyst activity by inhibiting the
1040:
The main difference between SMR and ATR is that SMR only uses air for combustion as a heat source to create steam, while ATR uses purified oxygen. The advantage of ATR is that the H
773:
medium pressures of 20-30 bar. High excess of steam is required, expressed by the (molar) steam-to-carbon (S/C) ratio. Typical S/C ratio values lie within the range 2.5:1 - 3:1.
1811:
859:
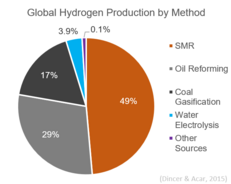
Globally, almost 50% of hydrogen is produced via steam reforming. It is currently the least expensive method for hydrogen production available in terms of its capital cost.
763:{\displaystyle \qquad \mathrm {CH} _{4}+2\,\mathrm {H} _{2}\mathrm {O} \rightleftharpoons \mathrm {CO} _{2}+4\,\mathrm {H} _{2}\qquad \Delta H_{DSR}=165\ \mathrm {kJ/mol} }
870:
produced from the process. Despite this, implementation of this technology remains problematic, costly, and increases the price of the produced hydrogen significantly.
452:{\displaystyle \qquad \mathrm {CH} _{4}+\mathrm {H} _{2}\mathrm {O} \rightleftharpoons \mathrm {CO} +3\,\mathrm {H} _{2}\qquad \Delta H_{SR}=206\ \mathrm {kJ/mol} }
605:{\displaystyle \qquad \mathrm {CO} +\mathrm {H} _{2}\mathrm {O} \rightleftharpoons \mathrm {CO} _{2}+\mathrm {H} _{2}\qquad \Delta H_{WGSR}=-41\ \mathrm {kJ/mol} }
2116:
1031:{\displaystyle \qquad \mathrm {CH} _{4}+0.5\,\mathrm {O} _{2}\rightleftharpoons \mathrm {CO} +2\,\mathrm {H} _{2}\qquad \Delta H_{R}=-24.5\ \mathrm {kJ/mol} }
614:
Some additional reactions occurring within steam reforming processes have been studied. Commonly the direct steam reforming (DSR) reaction is also included:
1846:
882:. The reaction takes place in a single chamber where the methane is partially oxidized. The reaction is exothermic. When the ATR uses carbon dioxide, the H
2291:
2090:
1257:(MCFC) do not have this problem, but operate at higher temperatures, slowing start-up time, and requiring costly materials and bulky insulation.
1774:
Reimert, Rainer; Marschner, Friedemann; Renner, Hans-Joachim; Boll, Walter; Supp, Emil; Brejc, Miron; Liebner, Waldemar; Schaub, Georg (2011).
1760:
1293:
890::CO ratio produced is 2.5:1. The outlet temperature of the syngas is between 950–1100 °C and outlet pressure can be as high as 100
2521:
2495:
1448:"Mathematical modelling and simulation of the thermo-catalytic decomposition of methane for economically improved hydrogen production"
2130:
1192:
becomes a possibility, which would prevent the release of carbon dioxide to the atmosphere, while adding to the cost of the process.
1212:
The reforming reaction takes place at high temperatures, making it slow to start up and requiring costly high-temperature materials.
797:
category. These reactors consist of an array of long and narrow tubes which are situated within the combustion chamber of a large
17:
2284:
1500:
2055:
1928:
1882:
1795:
1656:
1822:
86:
1951:"Explaining successful and failed investments in U.S. carbon capture and storage using empirical and expert assessments"
465:(WGSR), additional hydrogen is released by reaction of water with the carbon monoxide generated according to equation :
2597:
2506:
2277:
1736:
1391:
1354:
1989:
2076:
1218:
compounds in the fuel will poison certain catalysts, making it difficult to run this type of system from ordinary
2587:
2511:
2484:
2364:
2334:
238:, using low- or zero-carbon electricity. Zero carbon emissions 'turquoise' hydrogen is produced by one-step
2773:
866:(CCS) methods are being implemented within the industry, which have the potential to remove up to 90% of CO
817:
2003:"A Comparative Exergoeconomic Evaluation of the Synthesis Routes for Methanol Production from Natural Gas"
1447:
1408:
2778:
2369:
2094:
2035:
1908:
1339:
2727:
2666:
1847:"Fact of the Month May 2018: 10 Million Metric Tons of Hydrogen Produced Annually in the United States"
1344:
1254:
1189:
1134:
There is also interest in the development of much smaller units based on similar technology to produce
878:
Autothermal reforming (ATR) uses oxygen and carbon dioxide or steam in a reaction with methane to form
863:
223:
41:
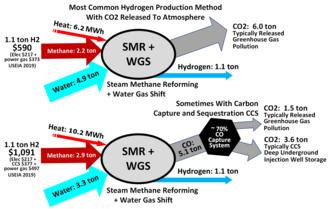
Illustrating inputs and outputs of steam reforming of natural gas, a process to produce hydrogen and CO
1684:
1604:
1409:"A review and comparative evaluation of thermochemical water splitting cycles for hydrogen production"
2793:
2722:
2707:
2440:
2324:
1084:
282:
2235:
2213:
1949:
Abdulla, Ahmed; Hanna, Ryan; Schell, Kristen R; Babacan, Oytun; Victor, David G (29 December 2020).
239:
2712:
2656:
2425:
2410:
2384:
2300:
2168:
1261:
462:
2435:
2420:
2405:
1864:
794:
1562:
1520:
317:
The name-giving reaction is the steam reforming (SR) reaction and is expressed by the equation:
2702:
2430:
2399:
2389:
2314:
2208:
1318:
1775:
1090:
Reforming for combustion engines is based on steam reforming, where non-methane hydrocarbons (
2614:
2581:
2450:
2222:
1308:
1250:
825:
1146:
are currently the subject of research and development, typically involving the reforming of
2752:
2682:
2672:
2609:
2545:
2359:
2329:
1298:
231:
8:
2737:
2732:
2687:
2646:
2576:
1496:
1288:
1265:
1249:(CO) produced by the reactor, making it necessary to include complex CO-removal systems.
77:
31:
2788:
2555:
2537:
2526:
2490:
2445:
2415:
1972:
1920:
1754:
1467:
1428:
1283:
798:
781:
250:
1620:
2783:
2602:
2479:
2195:
2051:
2047:
1976:
1924:
1791:
1742:
1732:
1704:
1662:
1652:
1624:
1582:
1540:
1471:
1432:
1387:
1329:
1323:
1242:
1057:
277:
2182:
2159:
1222:. Some new technologies have overcome this challenge with sulfur-tolerant catalysts.
2697:
2651:
2618:
2592:
2566:
2516:
2500:
2354:
2349:
2319:
2255:
2144:
2043:
2014:
1962:
1916:
1783:
1700:
1696:
1616:
1574:
1532:
1463:
1459:
1424:
1420:
1379:
2461:
2109:"Wärtsilä Launches GasReformer Product For Turning Oil Production Gas Into Energy"
2717:
2692:
2560:
2550:
2259:
1563:"Methane steam reforming, methanation and water-gas shift: I. Intrinsic kinetics"
1492:
1246:
281:
Depiction of the general process flow of a typical steam reforming plant. (PSA =
266:
65:
2131:"Method of operating a gas engine plant and fuel feeding system of a gas engine"
1685:"Review and evaluation of hydrogen production methods for better sustainability"
2641:
2623:
2394:
2374:
2339:
1967:
1950:
1313:
1303:
1111:
802:
227:
2161:
Hydrogen from
Exhaust Gas Fuel Reforming: Greener, Leaner and Smoother Engines
1746:
1666:
2767:
2379:
2344:
1787:
1708:
1628:
1586:
1544:
1234:
852:
842:
219:
215:
1887:
2742:
2661:
2269:
1726:
1646:
1196:
1185:
836:
235:
222:
when the carbon dioxide is (mostly) captured and stored geologically - see
1578:
1536:
1383:
289:
The purpose of pre-reforming is to break down higher hydrocarbons such as
2677:
2207:
Doty, F. David (2004). "A Realistic Look at
Hydrogen Price Projections".
1238:
poisoning by only chemisorbing sulfur rather than forming metal sulfides.
1180:
1163:
246:
234:, using solar thermal, low- or zero-carbon electricity or waste heat, or
201:
73:
69:
27:
Method for producing hydrogen and carbon monoxide from hydrocarbon fuels
2019:
2002:
2001:
Blumberg, Timo; Morosuk, Tatiana; Tsatsaronis, George (December 2017).
1143:
1139:
891:
790:
2747:
2470:
1080:
897:
In addition to reactions – , ATR introduces the following reaction:
821:
1883:"Vinder af VIDENSKABENS TOP 5: Hydrogen og methanol uden energifrås"
1605:"The kinetics of methane steam reforming over a Ni/α-Al2O catalyst"
1219:
1155:
1147:
1135:
1119:
829:
61:
2158:
Wyszynski, Miroslaw L.; Megaritis, Thanos; Lehrle, Roy S. (2001).
832:
shapes used are spoked wheels, gear wheels, and rings with holes (
2571:
1203:
1167:
1159:
1151:
1103:
809:
302:
298:
290:
218:
when the waste carbon dioxide is released to the atmosphere and
1349:
1278:
1225:
1215:
1095:
879:
813:
294:
262:
249:
produces most of the world's hydrogen. Hydrogen is used in the
57:
1208:
There are several challenges associated with this technology:
37:
1651:(2nd ed.). Cambridge, MA: Gulf Professional Publishing.
1126:) - thereby improving the fuel gas quality (methane number).
1376:
Hydrogen and Syngas
Production and Purification Technologies
1491:
1334:
1091:
2000:
1773:
2196:
Fossil fuel reforming not eliminating any carbon dioxides
1948:
886::CO ratio produced is 1:1; when the ATR uses steam, the H
309:), which allows for more efficient reforming downstream.
183:
117:
101:
76:
is the feedstock. The main purpose of this technology is
2157:
1870:(Report). United States Geological Survey. January 2020.
269:
catalysts, have been studied in detail since the 1950s.
1374:
Liu, Ke; Song, Chunshan; Subramani, Velu, eds. (2009).
261:
Steam reforming reaction kinetics, in particular using
144:
1812:"Hydrogen Production – Steam Methane Reforming (SMR)"
905:
622:
473:
325:
190:{\displaystyle {\ce {CH4 + H2O <=> CO + 3 H2}}}
89:
2167:(Technical report). Future Power Systems Group, The
1150:, but other fuels are also being considered such as
848:
Steam reforming of natural gas is 65–75% efficient.
1906:
80:. The reaction is represented by this equilibrium:
2248:
1268:) depending on the purity of the hydrogen product.
1030:
950:
762:
672:
604:
512:
451:
371:
189:
1066:
862:In an effort to decarbonise hydrogen production,
152:
151:
134:
133:
2765:
1373:
1907:Velazquez Abad, A.; Dodds, P. E. (2017-01-01),
214:Hydrogen produced by steam reforming is termed
2036:"FUELS – HYDROGEN STORAGE | Chemical Carriers"
1780:Ullmann's Encyclopedia of Industrial Chemistry
1611:. FRONTIERS IN CHEMICAL REACTION ENGINEERING.
1204:Challenges with reformers supplying fuel cells
1142:. Small-scale steam reforming units to supply
2285:
2040:Encyclopedia of Electrochemical Power Sources
1195:The cost of hydrogen production by reforming
2299:
2077:"Hydrogen Production: Natural Gas Reforming"
2033:
1728:Handbook of industrial hydrocarbon processes
1406:
845:which is advantageous for this application.
45:greenhouse gas that may be captured with CCS
1683:Dincer, Ibrahim; Acar, Canan (2015-09-14).
1294:Chemical looping reforming and gasification
2292:
2278:
1759:: CS1 maint: location missing publisher (
1682:
789:The reaction is conducted in multitubular
2212:
2042:, Amsterdam: Elsevier, pp. 504–518,
2018:
1966:
1603:Hou, Kaihu; Hughes, Ronald (2001-03-15).
1602:
1561:Xu, Jianguo; Froment, Gilbert F. (1989).
1560:
1518:
1075:
967:
937:
841:). Additionally, these shapes have a low
808:Inside the tubes, a mixture of steam and
696:
654:
388:
172:
2385:Environmentally healthy community design
1913:Encyclopedia of Sustainable Technologies
1689:International Journal of Hydrogen Energy
1521:"Kinetics of the methane-steam reaction"
1452:International Journal of Hydrogen Energy
1094:) of low quality gases are converted to
873:
780:
276:
36:
1880:
1874:
1724:
1644:
1445:
1407:Safari, Farid; Dincer, Ibrahim (2020).
1264:of the process is between 70% and 85% (
127:
14:
2766:
1915:, Oxford: Elsevier, pp. 293–304,
776:
2273:
1902:
1900:
1898:
1720:
1718:
2206:
2183:"Commonly used fuel reforming today"
1857:
1678:
1676:
1640:
1638:
1598:
1596:
1556:
1554:
1051:
785:Global Hydrogen Production by Method
24:
2522:waste-water treatment technologies
2252:Applied Catalysis B: Environmental
2034:Semelsberger, T. A. (2009-01-01),
1921:10.1016/b978-0-12-409548-9.10117-4
1895:
1881:Ramskov, Jens (16 December 2019).
1731:(Second ed.). Cambridge, MA.
1715:
1519:Akers, W. W.; Camp, D. P. (1955).
1024:
1021:
1018:
1010:
1007:
981:
970:
957:
954:
940:
921:
918:
756:
753:
750:
742:
739:
710:
699:
680:
677:
668:
657:
638:
635:
598:
595:
592:
584:
581:
546:
535:
520:
517:
508:
497:
488:
485:
445:
442:
439:
431:
428:
402:
391:
378:
375:
367:
356:
341:
338:
312:
25:
2805:
2507:agricultural wastewater treatment
1673:
1635:
1593:
1551:
1355:Timeline of hydrogen technologies
1245:membranes can be poisoned by the
1188:rather than distributed release,
1129:
2460:
2048:10.1016/b978-044452745-5.00331-2
1499:; Buchanan, Michelle V. (2004).
1413:Energy Conversion and Management
1173:
272:
2588:List of energy storage projects
2512:industrial wastewater treatment
2365:Environmental impact assessment
2335:Environmental impact assessment
2242:
2200:
2189:
2175:
2151:
2137:
2123:
2115:. 18 March 2013. Archived from
2101:
2083:
2069:
2027:
1994:
1983:
1942:
1911:, in Abraham, Martin A. (ed.),
1839:
1804:
1767:
980:
915:
709:
632:
545:
483:
401:
335:
251:industrial synthesis of ammonia
1955:Environmental Research Letters
1776:"Gas Production, 2. Processes"
1701:10.1016/j.ijhydene.2014.12.035
1512:
1485:
1464:10.1016/j.ijhydene.2021.11.057
1439:
1425:10.1016/j.enconman.2019.112182
1400:
1367:
1067:Steam reforming at small scale
912:
906:
816:catalyst. Catalysts with high
629:
623:
480:
474:
332:
326:
232:thermochemical water splitting
154:
129:
13:
1:
1621:10.1016/S1385-8947(00)00367-3
1360:
1072:range of 815 to 925 °C.
54:steam methane reforming (SMR)
2260:10.1016/j.apcatb.2014.05.044
1609:Chemical Engineering Journal
1063:reforming of the same fuel.
818:surface-area-to-volume ratio
812:are put into contact with a
256:
7:
2370:Environmental impact design
2038:, in Garche, Jürgen (ed.),
1340:Reformed methanol fuel cell
1272:
1255:molten carbonate fuel cells
793:reactors, a subtype of the
10:
2810:
2728:High-performance buildings
1725:Speight, James G. (2020).
1648:The refinery of the future
1645:Speight, James G. (2020).
1345:Reformer sponge iron cycle
1190:carbon capture and storage
1085:volatile organic compounds
1055:
864:carbon capture and storage
224:carbon capture and storage
56:is a method for producing
29:
2723:Heat recovery ventilation
2708:Environmental remediation
2633:
2536:
2469:
2458:
2325:Climate smart agriculture
2307:
1184:of carbon dioxide into a
820:are preferred because of
283:Pressure swing adsorption
200:The reaction is strongly
2713:Glass in green buildings
2657:sustainable architecture
2301:Environmental technology
2169:University of Birmingham
1968:10.1088/1748-9326/abd19e
1909:"Production of Hydrogen"
1865:Nitrogen (Fixed)—Ammonia
1788:10.1002/14356007.o12_o01
1262:thermodynamic efficiency
824:limitations due to high
463:water-gas shift reaction
30:Not to be confused with
2145:"Fossil fuel processor"
2091:"Atmospheric Emissions"
1497:Dresselhaus, Mildred S.
1446:Lumbers, Brock (2022).
805:terrace wall reformer.
18:Steam methane reforming
2703:Environmental movement
2400:Sustainability science
2390:Public interest design
2315:Appropriate technology
2230:Cite journal requires
1319:Lane hydrogen producer
1251:Solid oxide fuel cells
1076:For combustion engines
1032:
786:
764:
606:
453:
286:
191:
46:
2582:hydrogen technologies
2496:Solid waste treatment
1579:10.1002/aic.690350109
1537:10.1002/aic.690010415
1384:10.1002/9780470561256
1309:Hydrogen technologies
1102:+ CO) and finally to
1033:
874:Autothermal reforming
826:operating temperature
784:
765:
607:
454:
280:
253:and other chemicals.
192:
72:with water. Commonly
40:
2753:Water heat recycling
2683:Efficient energy use
2673:Conservation biology
2610:Sustainable lighting
2546:Efficient energy use
2360:Environmental Design
2330:Environmental design
2254:. 160–161: 525–533.
1502:The Hydrogen Economy
1299:Cracking (chemistry)
903:
620:
471:
323:
87:
2774:Hydrogen production
2738:Nature conservation
2733:Land rehabilitation
2688:Energy conservation
2577:carbon-neutral fuel
2485:dispersion modeling
1819:Hydrogen Fact Sheet
1508:(Technical report).
1493:Crabtree, George W.
1289:Catalytic reforming
1138:as a feedstock for
777:Industrial practice
285:, NG = Natural gas)
245:Steam reforming of
185:
140:
119:
103:
78:hydrogen production
32:catalytic reforming
2779:Chemical processes
2556:Energy development
2538:Sustainable energy
2527:water purification
2491:Industrial ecology
2020:10.3390/app7121213
1828:on 4 February 2006
1284:Boudouard reaction
1028:
799:industrial furnace
787:
760:
602:
449:
287:
187:
173:
159:
107:
91:
47:
2761:
2760:
2598:commercialization
2057:978-0-444-52745-5
1930:978-0-12-804792-7
1797:978-3-527-30673-2
1658:978-0-12-816995-7
1330:Partial oxidation
1324:Methane pyrolysis
1243:polymer fuel cell
1058:Partial oxidation
1052:Partial oxidation
1005:
795:plug flow reactor
737:
579:
426:
240:methane pyrolysis
176:
165:
161:
122:
110:
94:
68:) by reaction of
16:(Redirected from
2801:
2794:Industrial gases
2698:Energy recycling
2619:electric vehicle
2593:Renewable energy
2567:alternative fuel
2517:sewage treatment
2501:Waste management
2464:
2355:Energy recycling
2350:Electric vehicle
2320:Clean technology
2294:
2287:
2280:
2271:
2270:
2264:
2263:
2246:
2240:
2239:
2233:
2228:
2226:
2218:
2216:
2204:
2198:
2193:
2187:
2186:
2179:
2173:
2172:
2166:
2155:
2149:
2148:
2141:
2135:
2134:
2127:
2121:
2120:
2105:
2099:
2098:
2093:. Archived from
2087:
2081:
2080:
2073:
2067:
2066:
2065:
2064:
2031:
2025:
2024:
2022:
2007:Applied Sciences
1998:
1992:
1987:
1981:
1980:
1970:
1946:
1940:
1939:
1938:
1937:
1904:
1893:
1892:
1878:
1872:
1871:
1869:
1861:
1855:
1854:
1843:
1837:
1836:
1835:
1833:
1827:
1821:, archived from
1816:
1808:
1802:
1801:
1771:
1765:
1764:
1758:
1750:
1722:
1713:
1712:
1680:
1671:
1670:
1642:
1633:
1632:
1600:
1591:
1590:
1558:
1549:
1548:
1516:
1510:
1509:
1507:
1489:
1483:
1482:
1480:
1478:
1458:(7): 4265–4283.
1443:
1437:
1436:
1404:
1398:
1397:
1371:
1241:Low temperature
1037:
1035:
1034:
1029:
1027:
1017:
1003:
993:
992:
979:
978:
973:
960:
949:
948:
943:
930:
929:
924:
769:
767:
766:
761:
759:
749:
735:
728:
727:
708:
707:
702:
689:
688:
683:
671:
666:
665:
660:
647:
646:
641:
611:
609:
608:
603:
601:
591:
577:
567:
566:
544:
543:
538:
529:
528:
523:
511:
506:
505:
500:
491:
458:
456:
455:
450:
448:
438:
424:
417:
416:
400:
399:
394:
381:
370:
365:
364:
359:
350:
349:
344:
242:of natural gas.
228:'green' hydrogen
196:
194:
193:
188:
186:
184:
181:
174:
163:
162:
160:
158:
157:
150:
142:
141:
139:
132:
124:
120:
118:
115:
108:
102:
99:
92:
21:
2809:
2808:
2804:
2803:
2802:
2800:
2799:
2798:
2764:
2763:
2762:
2757:
2718:Green computing
2693:Energy recovery
2629:
2561:Energy recovery
2551:Electrification
2532:
2478:Air pollution (
2465:
2456:
2303:
2298:
2268:
2267:
2247:
2243:
2231:
2229:
2220:
2219:
2214:10.1.1.538.3537
2205:
2201:
2194:
2190:
2181:
2180:
2176:
2164:
2156:
2152:
2143:
2142:
2138:
2129:
2128:
2124:
2107:
2106:
2102:
2089:
2088:
2084:
2075:
2074:
2070:
2062:
2060:
2058:
2032:
2028:
1999:
1995:
1988:
1984:
1947:
1943:
1935:
1933:
1931:
1905:
1896:
1879:
1875:
1867:
1863:
1862:
1858:
1845:
1844:
1840:
1831:
1829:
1825:
1814:
1810:
1809:
1805:
1798:
1772:
1768:
1752:
1751:
1739:
1723:
1716:
1681:
1674:
1659:
1643:
1636:
1601:
1594:
1559:
1552:
1517:
1513:
1505:
1490:
1486:
1476:
1474:
1444:
1440:
1405:
1401:
1394:
1372:
1368:
1363:
1275:
1247:carbon monoxide
1232:
1206:
1176:
1132:
1125:
1117:
1109:
1101:
1078:
1069:
1060:
1054:
1043:
1013:
1006:
988:
984:
974:
969:
968:
953:
944:
939:
938:
925:
917:
916:
904:
901:
900:
889:
885:
876:
869:
779:
745:
738:
717:
713:
703:
698:
697:
684:
676:
675:
667:
661:
656:
655:
642:
634:
633:
621:
618:
617:
587:
580:
553:
549:
539:
534:
533:
524:
516:
515:
507:
501:
496:
495:
484:
472:
469:
468:
434:
427:
409:
405:
395:
390:
389:
374:
366:
360:
355:
354:
345:
337:
336:
324:
321:
320:
315:
313:Steam reforming
308:
275:
259:
230:is produced by
220:'blue' hydrogen
216:'grey' hydrogen
211:= 206 kJ/mol).
210:
182:
177:
153:
146:
145:
143:
135:
128:
126:
125:
123:
116:
111:
100:
95:
90:
88:
85:
84:
66:carbon monoxide
50:Steam reforming
44:
35:
28:
23:
22:
15:
12:
11:
5:
2807:
2797:
2796:
2791:
2786:
2781:
2776:
2759:
2758:
2756:
2755:
2750:
2745:
2740:
2735:
2730:
2725:
2720:
2715:
2710:
2705:
2700:
2695:
2690:
2685:
2680:
2675:
2670:
2664:
2659:
2654:
2649:
2644:
2637:
2635:
2631:
2630:
2628:
2627:
2624:hybrid vehicle
2621:
2615:Transportation
2612:
2607:
2606:
2605:
2600:
2590:
2585:
2579:
2574:
2569:
2563:
2558:
2553:
2548:
2542:
2540:
2534:
2533:
2531:
2530:
2524:
2519:
2514:
2509:
2503:
2498:
2493:
2488:
2482:
2475:
2473:
2467:
2466:
2459:
2457:
2455:
2454:
2448:
2443:
2438:
2433:
2428:
2423:
2418:
2413:
2408:
2402:
2397:
2395:Sustainability
2392:
2387:
2382:
2377:
2375:Green building
2372:
2367:
2362:
2357:
2352:
2347:
2342:
2340:Eco-innovation
2337:
2332:
2327:
2322:
2317:
2311:
2309:
2305:
2304:
2297:
2296:
2289:
2282:
2274:
2266:
2265:
2241:
2232:|journal=
2199:
2188:
2174:
2150:
2136:
2122:
2119:on 2015-05-11.
2113:Marine Insight
2100:
2097:on 2013-09-26.
2082:
2068:
2056:
2026:
1993:
1982:
1941:
1929:
1894:
1873:
1856:
1838:
1803:
1796:
1766:
1737:
1714:
1672:
1657:
1634:
1615:(1): 311–328.
1592:
1550:
1531:(4): 471–475.
1511:
1484:
1438:
1399:
1392:
1365:
1364:
1362:
1359:
1358:
1357:
1352:
1347:
1342:
1337:
1332:
1327:
1326:(for Hydrogen)
1321:
1316:
1314:Industrial gas
1311:
1306:
1304:Hydrogen pinch
1301:
1296:
1291:
1286:
1281:
1274:
1271:
1270:
1269:
1258:
1239:
1230:
1223:
1213:
1205:
1202:
1175:
1172:
1131:
1130:For fuel cells
1128:
1123:
1115:
1112:carbon dioxide
1107:
1099:
1077:
1074:
1068:
1065:
1056:Main article:
1053:
1050:
1041:
1026:
1023:
1020:
1016:
1012:
1009:
1002:
999:
996:
991:
987:
983:
977:
972:
966:
963:
959:
956:
952:
947:
942:
936:
933:
928:
923:
920:
914:
911:
908:
887:
883:
875:
872:
867:
828:. Examples of
803:Foster-Wheeler
778:
775:
758:
755:
752:
748:
744:
741:
734:
731:
726:
723:
720:
716:
712:
706:
701:
695:
692:
687:
682:
679:
674:
670:
664:
659:
653:
650:
645:
640:
637:
631:
628:
625:
600:
597:
594:
590:
586:
583:
576:
573:
570:
565:
562:
559:
556:
552:
548:
542:
537:
532:
527:
522:
519:
514:
510:
504:
499:
494:
490:
487:
482:
479:
476:
447:
444:
441:
437:
433:
430:
423:
420:
415:
412:
408:
404:
398:
393:
387:
384:
380:
377:
373:
369:
363:
358:
353:
348:
343:
340:
334:
331:
328:
314:
311:
306:
274:
271:
258:
255:
226:. Zero carbon
208:
198:
197:
180:
171:
168:
156:
149:
138:
131:
114:
106:
98:
42:
26:
9:
6:
4:
3:
2:
2806:
2795:
2792:
2790:
2787:
2785:
2782:
2780:
2777:
2775:
2772:
2771:
2769:
2754:
2751:
2749:
2746:
2744:
2741:
2739:
2736:
2734:
2731:
2729:
2726:
2724:
2721:
2719:
2716:
2714:
2711:
2709:
2706:
2704:
2701:
2699:
2696:
2694:
2691:
2689:
2686:
2684:
2681:
2679:
2676:
2674:
2671:
2668:
2667:New Classical
2665:
2663:
2660:
2658:
2655:
2653:
2650:
2648:
2645:
2643:
2639:
2638:
2636:
2632:
2625:
2622:
2620:
2616:
2613:
2611:
2608:
2604:
2601:
2599:
2596:
2595:
2594:
2591:
2589:
2586:
2583:
2580:
2578:
2575:
2573:
2570:
2568:
2564:
2562:
2559:
2557:
2554:
2552:
2549:
2547:
2544:
2543:
2541:
2539:
2535:
2528:
2525:
2523:
2520:
2518:
2515:
2513:
2510:
2508:
2504:
2502:
2499:
2497:
2494:
2492:
2489:
2486:
2483:
2481:
2477:
2476:
2474:
2472:
2468:
2463:
2452:
2449:
2447:
2444:
2442:
2441:refurbishment
2439:
2437:
2434:
2432:
2429:
2427:
2424:
2422:
2419:
2417:
2414:
2412:
2409:
2407:
2404:Sustainable (
2403:
2401:
2398:
2396:
2393:
2391:
2388:
2386:
2383:
2381:
2380:Green vehicle
2378:
2376:
2373:
2371:
2368:
2366:
2363:
2361:
2358:
2356:
2353:
2351:
2348:
2346:
2345:Ecotechnology
2343:
2341:
2338:
2336:
2333:
2331:
2328:
2326:
2323:
2321:
2318:
2316:
2313:
2312:
2310:
2306:
2302:
2295:
2290:
2288:
2283:
2281:
2276:
2275:
2272:
2261:
2257:
2253:
2245:
2237:
2224:
2215:
2210:
2203:
2197:
2192:
2185:. 2000-10-04.
2184:
2178:
2170:
2163:
2162:
2154:
2147:. 2000-10-04.
2146:
2140:
2132:
2126:
2118:
2114:
2110:
2104:
2096:
2092:
2086:
2078:
2072:
2059:
2053:
2049:
2045:
2041:
2037:
2030:
2021:
2016:
2012:
2008:
2004:
1997:
1991:
1986:
1978:
1974:
1969:
1964:
1961:(1): 014036.
1960:
1956:
1952:
1945:
1932:
1926:
1922:
1918:
1914:
1910:
1903:
1901:
1899:
1890:
1889:
1884:
1877:
1866:
1860:
1852:
1848:
1842:
1824:
1820:
1813:
1807:
1799:
1793:
1789:
1785:
1781:
1777:
1770:
1762:
1756:
1748:
1744:
1740:
1738:9780128099230
1734:
1730:
1729:
1721:
1719:
1710:
1706:
1702:
1698:
1695:(34): 11096.
1694:
1690:
1686:
1679:
1677:
1668:
1664:
1660:
1654:
1650:
1649:
1641:
1639:
1630:
1626:
1622:
1618:
1614:
1610:
1606:
1599:
1597:
1588:
1584:
1580:
1576:
1572:
1568:
1567:AIChE Journal
1564:
1557:
1555:
1546:
1542:
1538:
1534:
1530:
1526:
1525:AIChE Journal
1522:
1515:
1504:
1503:
1498:
1494:
1488:
1473:
1469:
1465:
1461:
1457:
1453:
1449:
1442:
1434:
1430:
1426:
1422:
1418:
1414:
1410:
1403:
1395:
1393:9780470561256
1389:
1385:
1381:
1377:
1370:
1366:
1356:
1353:
1351:
1348:
1346:
1343:
1341:
1338:
1336:
1333:
1331:
1328:
1325:
1322:
1320:
1317:
1315:
1312:
1310:
1307:
1305:
1302:
1300:
1297:
1295:
1292:
1290:
1287:
1285:
1282:
1280:
1277:
1276:
1267:
1263:
1259:
1256:
1252:
1248:
1244:
1240:
1236:
1235:chemisorption
1227:
1224:
1221:
1217:
1214:
1211:
1210:
1209:
1201:
1198:
1193:
1191:
1187:
1182:
1179:is an issue.
1174:Disadvantages
1171:
1169:
1165:
1161:
1157:
1153:
1149:
1145:
1141:
1137:
1127:
1121:
1113:
1105:
1097:
1096:synthesis gas
1093:
1088:
1086:
1082:
1073:
1064:
1059:
1049:
1047:
1038:
1014:
1000:
997:
994:
989:
985:
975:
964:
961:
945:
934:
931:
926:
909:
898:
895:
893:
881:
871:
865:
860:
857:
854:
853:United States
849:
846:
844:
843:pressure drop
840:
839:
838:Raschig rings
835:
831:
827:
823:
819:
815:
811:
806:
804:
800:
796:
792:
783:
774:
770:
746:
732:
729:
724:
721:
718:
714:
704:
693:
690:
685:
662:
651:
648:
643:
626:
615:
612:
588:
574:
571:
568:
563:
560:
557:
554:
550:
540:
530:
525:
502:
492:
477:
466:
464:
459:
435:
421:
418:
413:
410:
406:
396:
385:
382:
361:
351:
346:
329:
318:
310:
304:
300:
296:
292:
284:
279:
273:Pre-reforming
270:
268:
264:
254:
252:
248:
243:
241:
237:
233:
229:
225:
221:
217:
212:
207:
203:
178:
169:
166:
147:
136:
112:
104:
96:
83:
82:
81:
79:
75:
71:
67:
63:
59:
55:
51:
39:
33:
19:
2743:Permaculture
2662:New Urbanism
2634:Conservation
2426:food systems
2411:architecture
2251:
2244:
2223:cite journal
2202:
2191:
2177:
2160:
2153:
2139:
2125:
2117:the original
2112:
2103:
2095:the original
2085:
2071:
2061:, retrieved
2039:
2029:
2013:(12): 1213.
2010:
2006:
1996:
1985:
1958:
1954:
1944:
1934:, retrieved
1912:
1891:(in Danish).
1886:
1876:
1859:
1850:
1841:
1830:, retrieved
1823:the original
1818:
1806:
1779:
1769:
1727:
1692:
1688:
1647:
1612:
1608:
1573:(1): 88–96.
1570:
1566:
1528:
1524:
1514:
1501:
1487:
1475:. Retrieved
1455:
1451:
1441:
1416:
1412:
1402:
1375:
1369:
1207:
1197:fossil fuels
1194:
1186:point source
1177:
1133:
1089:
1079:
1070:
1061:
1045:
1039:
899:
896:
877:
861:
858:
850:
847:
837:
833:
807:
788:
771:
616:
613:
467:
460:
319:
316:
288:
260:
244:
236:electrolysis
213:
205:
199:
70:hydrocarbons
53:
49:
48:
2678:Ecoforestry
2436:procurement
2421:development
2406:agriculture
1253:(SOFC) and
1181:Fossil fuel
1164:diesel fuel
1083:and vented
247:natural gas
202:endothermic
74:natural gas
2768:Categories
2647:insulation
2640:Building (
2603:transition
2446:technology
2431:industries
2063:2021-11-16
1990:Topsoe ATR
1936:2021-11-16
1888:Ingeniøren
1851:Energy.gov
1747:1129385226
1667:1179046717
1419:: 112182.
1361:References
1144:fuel cells
1140:fuel cells
1081:Flared gas
791:packed bed
2789:Catalysis
2748:Recycling
2471:Pollution
2451:transport
2209:CiteSeerX
1977:234429781
1832:28 August
1755:cite book
1709:0360-3199
1629:1385-8947
1587:1547-5905
1545:1547-5905
1472:244814932
1433:214089650
1266:LHV basis
998:−
982:Δ
951:⇌
822:diffusion
711:Δ
673:⇌
572:−
547:Δ
513:⇌
403:Δ
372:⇌
257:Reactions
155:⇀
148:−
137:−
130:↽
2784:Fuel gas
1477:16 March
1273:See also
1220:gasoline
1156:gasoline
1148:methanol
1136:hydrogen
1120:hydrogen
830:catalyst
461:Via the
62:hydrogen
2652:natural
2572:biofuel
2505:Water (
2480:control
2308:General
1168:ethanol
1160:autogas
1152:propane
1104:methane
810:methane
303:methane
299:naphtha
291:propane
267:alumina
2565:Fuel (
2416:design
2211:
2054:
1975:
1927:
1794:
1745:
1735:
1707:
1665:
1655:
1627:
1585:
1543:
1470:
1431:
1390:
1350:Syngas
1279:Biogas
1226:Coking
1216:Sulfur
1166:, and
1118:) and
1048:= 0).
1004:
880:syngas
814:nickel
736:
578:
425:
295:butane
263:nickel
58:syngas
2642:green
2165:(PDF)
1973:S2CID
1868:(PDF)
1826:(PDF)
1815:(PDF)
1506:(PDF)
1468:S2CID
1429:S2CID
1092:NMHCs
301:into
2236:help
2052:ISBN
1925:ISBN
1834:2014
1792:ISBN
1761:link
1743:OCLC
1733:ISBN
1705:ISSN
1663:OCLC
1653:ISBN
1625:ISSN
1583:ISSN
1541:ISSN
1479:2022
1388:ISBN
1335:PROX
1260:The
1001:24.5
851:The
834:see:
64:and
2256:doi
2044:doi
2015:doi
1963:doi
1917:doi
1784:doi
1697:doi
1617:doi
1575:doi
1533:doi
1460:doi
1421:doi
1417:205
1380:doi
1114:(CO
1110:),
1106:(CH
935:0.5
892:bar
733:165
422:206
305:(CH
297:or
52:or
2770::
2227::
2225:}}
2221:{{
2111:.
2050:,
2009:.
2005:.
1971:.
1959:16
1957:.
1953:.
1923:,
1897:^
1885:.
1849:.
1817:,
1790:.
1782:.
1778:.
1757:}}
1753:{{
1741:.
1717:^
1703:.
1693:40
1691:.
1687:.
1675:^
1661:.
1637:^
1623:.
1613:82
1607:.
1595:^
1581:.
1571:35
1569:.
1565:.
1553:^
1539:.
1527:.
1523:.
1495:;
1466:.
1456:47
1454:.
1450:.
1427:.
1415:.
1411:.
1386:.
1378:.
1170:.
1162:,
1158:,
1154:,
1122:(H
1098:(H
894:.
575:41
293:,
209:SR
204:(Δ
164:CO
93:CH
2669:)
2626:)
2617:(
2584:)
2529:)
2487:)
2453:)
2293:e
2286:t
2279:v
2262:.
2258::
2238:)
2234:(
2217:.
2171:.
2133:.
2079:.
2046::
2023:.
2017::
2011:7
1979:.
1965::
1919::
1853:.
1800:.
1786::
1763:)
1749:.
1711:.
1699::
1669:.
1631:.
1619::
1589:.
1577::
1547:.
1535::
1529:1
1481:.
1462::
1435:.
1423::
1396:.
1382::
1231:2
1124:2
1116:2
1108:4
1100:2
1046:H
1042:2
1025:l
1022:o
1019:m
1015:/
1011:J
1008:k
995:=
990:R
986:H
976:2
971:H
965:2
962:+
958:O
955:C
946:2
941:O
932:+
927:4
922:H
919:C
913:]
910:4
907:[
888:2
884:2
868:2
757:l
754:o
751:m
747:/
743:J
740:k
730:=
725:R
722:S
719:D
715:H
705:2
700:H
694:4
691:+
686:2
681:O
678:C
669:O
663:2
658:H
652:2
649:+
644:4
639:H
636:C
630:]
627:3
624:[
599:l
596:o
593:m
589:/
585:J
582:k
569:=
564:R
561:S
558:G
555:W
551:H
541:2
536:H
531:+
526:2
521:O
518:C
509:O
503:2
498:H
493:+
489:O
486:C
481:]
478:2
475:[
446:l
443:o
440:m
436:/
432:J
429:k
419:=
414:R
411:S
407:H
397:2
392:H
386:3
383:+
379:O
376:C
368:O
362:2
357:H
352:+
347:4
342:H
339:C
333:]
330:1
327:[
307:4
265:-
206:H
179:2
175:H
170:3
167:+
121:O
113:2
109:H
105:+
97:4
60:(
43:2
34:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.