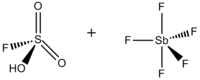919:, cannot be depicted using only two-electron, two-center bonds, and require, instead, two-electron, three (or more) center bonding. In these ions, two electrons are delocalized over more than two atoms, rendering these bond centers so electron deficient that they enable saturated alkanes to participate in electrophilic reactions. The discovery of hypercoordinated carbocations fueled the nonclassical ion controversy of the 1950s and 60s. Due to the slow timescale of H-NMR, the rapidly equilibrating positive charges on hydrogen atoms would likely go undetected. However,
200:
145:
1044:
374:
369:
364:
379:
808:
359:
40:
354:
550:
1022:
27:
1073:
612:
555:
1207:
Commeyras, A.; Olah, G. A. (1969). "Chemistry in Super Acids. II. Nuclear
Magnetic Resonance and Laser Raman Spectroscopic Study of the Antimony Pentafluoride-Fluorosulfuric Acid (Sulfur Dioxide) Solvent System ("Magic Acid"). The Effect of Added Halides, Water, Alcohols, and Carboxylic Acids. Study
1050:
Magic acid also catalyzes electrophilic hydroxylation of aromatic compounds with hydrogen peroxide, resulting in high-yield preparation of monohydroxylated products. Phenols exist as completely protonated species in superacid solutions, and when produced in the reaction, are then deactivated toward
898:
because it contains only two-electron, single bonds to adjacent carbon atoms. Many tertiary cycloalkyl cations can also be formed in superacidic solutions. One such example is the 1-methyl-1-cyclopentyl cation, which is formed from both the cyclopentane and cyclohexane precursor. In the case of the
893:
Magic acid has low nucleophilicity, allowing for increased stability of carbocations in solution. The "classical" trivalent carbocation can be observed in the acid medium, and has been found to be planar and sp-hybridized. Because the carbon atom is surrounded by only six valence electrons, it is
1087:
As with all strong acids, and especially superacids, proper personal protective equipment should be used. In addition to the obligatory gloves and goggles, the use of a faceshield and full-face respirator are also recommended. Predictably, magic acid is highly toxic upon ingestion and inhalation,
1028:
It is on this note that George Olah suggests we no longer take as synonymous the names "alkane" and "paraffin." The word "paraffin" is derived from the Latin "parum affinis", meaning "lacking in affinity." He says, "It is, however, with some nostalgia that we make this recommendation, as ‘inert
1038:
determined that the mechanism depends on the amount of magic acid used. Near molar equivalency, only O–O cleavage is observed, but with increasing excess of magic acid, C–O cleavage competes with O–O cleavage. The excess acid likely deactivates the hydrogen peroxide formed in C–O heterolysis.
1037:
Magic acid catalyzes cleavage-rearrangement reactions of tertiary hydroperoxides and tertiary alcohols. The nature of the experiments used to determine the mechanism, namely the fact that they took place in superacid medium, allowed observation of the carbocation intermediates formed. It was
1015:
Larger alkanes, such as ethane, are also reactive in magic acid, and both exchange hydrogen atoms and condense to form larger carbocations, such as protonated neopentane. This ion is then cloven at higher temperatures, and reacts to release hydrogen gas and forms the t-amyl cation at lower
556:
553:
939:
The bridging methylene carbon atom is pentacoordinated, with three two-electron, two-center bonds, and one two-electron, three-center bond with its remaining sp orbital. Quantum mechanical calculations have also shown that the classical model is not an energy minimum.
821:
In the above figure, Equilibrium I accounts for 80% of the NMR data, while
Equilibrium II accounts for about 20%. As the ratio of the two compounds increases from 0.4–1.4, new NMR signals appear and increase in intensity with increasing concentrations of
765:, and was to be used to study stable carbocations. Gillespie also used the acid system to generate electron-deficient inorganic cations. The name originated after a Christmas party in 1966, when a member of the Olah lab placed a
509:
1369:
Olah, G. A.; Yonena, N.; Ohnishi, R (1976). "Oxyfunctionalization of hydrocarbons. 6. Electrophilic oxygenation of aliphatic alcohols, ketones, and aldehydes with ozone in superacids. Preparation of bifunctional derivatives".
1067:. The mechanism is similar to that of protolysis of alkanes, with an electrophilic insertion into the single σ bonds of the alkane. The hydrocarbon–ozone complex transition state has the form of a penta-coordinated ion.
903:, the cyclopentyl cation is formed from isomerization of the secondary carbocation to the tertiary, more stable carbocation. Cyclopropylcarbenium ions, alkenyl cations, and arenium cations have also been observed.
773:-butyl cation, suggesting that the paraffin chain that forms the wax had been cleaved, then isomerized into the relatively stable tertiary carbocation. The name appeared in a paper published by the Olah lab.
387:
1315:
Olah, G. A.; Parker, D. G.; Yoneda, Y.; Pelizza, F. (1976). "Oxyfunctionalization of
Hydrocarbons. 1. Protolytic Cleavage-Rearrangement Reactions of Tertiary Alkyl Hydroperoxides with Magic Acid".
334:
505:
1342:
Olah, G. A.; Ohnishi, R. (1978). "Oxyfunctionalization of hydrocarbons. 8. Electrophilic hydroxylation of benzene, alkylbenzenes, and halobenzenes with hydrogen peroxide in superacids".
1009:
909:
554:
933:
927:, and C NMR have been used to investigate bridged carbocation systems. One controversial cation, the norbornyl cation, has been observed in several media, Magic acid among them.
964:
ion at 140 °C and atmospheric pressure, though some hydrocarbon ions of greater molecular weights are also formed as byproducts. Hydrogen gas is another reaction byproduct.
814:(In both of these structures, the sulfur has tetrahedral coordination, not planar. The double bonds between sulfur and oxygen are more properly represented as single bonds, with
1397:
Olah, G. A.; Yoneda, N.; Parker, D. G. (1976). "Oxyfunctionalization of hydrocarbons. 3. Superacid catalyzed oxygenation of alkanes with ozone involving protonated ozone, O
1029:
gases’ at least maintained their ‘nobility’ as their chemical reactivity became apparent, but referring to ‘noble hydrocarbons’ would seem to be inappropriate."
915:
As use of the Magic acid system became more widespread, however, higher-coordinate carbocations were observed. Penta-coordinate carbocations, also described as
501:
578:
625:
818:
negative charges on the oxygen atoms and a formal plus two charge on the sulfur. The antimony atoms will also have a formal charge of minus one.)
761:, and found the solution to be several million times more acidic than sulfuric acid alone. The magic acid system was developed in the 1960s by
249:
102:
1268:
Olah, G. A.; Schlosberg, R. H. (1968). "Chemistry in Super Acids. I. Hydrogen
Exchange and Polycondensation of Methane and Alkanes in FSO
979:. This is evidence to suggest that in these reactions, methane is indeed a base, and can accept a proton from the acid medium to form
834:
All proton-producing acids stronger than 100% sulfuric acid are considered superacids, and are characterized by low values of the
1191:
794:
best generates carbonium ions, the effects of the system at other molar ratios have also been documented. When the ratio SbF
802:
F is less than 0.2, the following two equilibria, determined by F NMR spectroscopy, are the most prominent in solution:
620:
214:
233:
InChI=1/FHO3S.5FH.Sb/c1-5(2,3)4;;;;;;/h(H,2,3,4);5*1H;/q;;;;;;+5/p-5/rF5Sb.FHO3S/c1-6(2,3,4)5;1-5(2,3)4/h;(H,2,3,4)
769:
candle into the acid, and found that it dissolved quite rapidly. Examination of the solution with H-NMR showed a
632:
691:
585:
1439:
315:
1003:– the carbonium ion. This species is quite reactive, and can yield several new carbocations, shown below.
975:
H, methane has been shown to interchange hydrogen atoms for deuterium atoms, and HD is released rather than H
826:. The resolution of the signals decreases as well, because of the increasing viscosity of the liquid system.
564:
1159:
455:
140:
378:
178:
991:. This ion is then deprotonated, explaining the hydrogen exchange, or loses a hydrogen molecule to form
485:
373:
1288:
and
Related Hydrocarbon Ions.The High Chemical Reactivity of "Paraffins" in Ionic Solution Reactions".
363:
493:
1449:
807:
195:
835:
521:
368:
1444:
671:
1181:
1051:
further electrophilic attack. Protonated hydrogen peroxide is the active hydroxylating agent.
353:
122:
706:
401:
346:
52:
705:
in liquid media. Magic acid and other superacids are also used to catalyze isomerization of
1097:
895:
870:
358:
78:
497:
8:
1434:
758:
738:
663:
68:
199:
144:
924:
726:
687:
1123:
Olah, G. A. (2005). "Crossing
Conventional Boundaries in Half a Century of Research".
1187:
1140:
699:
166:
1079:
Alcohols, ketones, and aldehydes are oxygenated by electrophilic insertion as well.
861:, has a Hammett acidity function, of −13, and that of the 1:1 magic acid system, HSO
1410:
1379:
1351:
1324:
1297:
1247:
1217:
1132:
916:
272:
679:
439:
1043:
920:
854:
754:
742:
473:
603:
465:
1428:
1314:
815:
702:
477:
443:
133:
1251:
1144:
766:
695:
577:
411:
39:
900:
762:
710:
584:
517:
1414:
1383:
1355:
1328:
1301:
1276:("Magic Acid") Solution. Protonation of Alkanes and the Intermediacy of
1221:
563:
461:
894:
highly electron deficient and electrophilic. It is easily described by
683:
570:
291:
113:
1136:
513:
1021:
1008:
908:
659:
447:
423:
223:
InChI=1S/FHO3S.5FH.Sb/c1-5(2,3)4;;;;;;/h(H,2,3,4);5*1H;/q;;;;;;+5/p-5
26:
753:
to form salts in nonaqueous solution. The term itself was coined by
602:
Except where otherwise noted, data are given for materials in their
1179:
1072:
932:
873:, the strongest known superacid, is believed to reach extrapolated
750:
722:
536:
1396:
529:
101:
949:
714:
571:
469:
153:
1238:
1088:
causes severe skin and eye burns, and is toxic to aquatic life.
662:
consisting of a mixture, most commonly in a 1:1 molar ratio, of
1368:
746:
481:
16:
Superacid system prepared from a Brønsted and a Lewis superacid
427:
1064:
718:
91:
1267:
407:
183:
1186:(2nd ed.). New York: John Wiley and Sons. p. 49.
1059:
Oxygenation of alkanes can be catalyzed by a magic acid–SO
489:
329:
Extremely corrosive, toxic, violent hydrolysis, oxidizer.
1180:
Olah, G. A.; Prakash, S.; Molnar, A.; Sommer, J. (2009).
1206:
415:
1341:
888:
435:
686:superacid system was developed in the 1960s by the
1160:"A Basic History of Acid—From Aristotle to Arnold"
419:
56:Sulfurofluoridic acid — pentafluorostiborane (1:1)
737:The term "superacid" was first used in 1927 when
1426:
1032:
757:later, after Conant combined sulfuric acid with
165:
1118:
1116:
1114:
552:
77:
1233:
1231:
525:
431:
32:Fluorosulfuric acid-antimony pentafluoride 1:1
1157:
1111:
1237:
1228:
1122:
952:. For instance, methane reacts to form the
198:
143:
121:
1263:
1261:
943:
1403:Journal of the American Chemical Society
1372:Journal of the American Chemical Society
1317:Journal of the American Chemical Society
1290:Journal of the American Chemical Society
1210:Journal of the American Chemical Society
1240:Angewandte Chemie International Edition
1054:
194:
1427:
1258:
134:
948:Magic acid is capable of protonating
226:Key: QNDPUZFBWUBSNH-UHFFFAOYSA-I
889:Observations of stable carbocations
236:Key: QNDPUZFBWUBSNH-UDIXYDQRAK
156:
13:
846:, has a Hammett acidity function,
548:
14:
1461:
786:Although a 1:1 molar ratio of HSO
694:, and has been used to stabilize
1071:
1063:ClF solution in the presence of
1042:
1020:
1007:
931:
907:
838:. For instance, sulfuric acid, H
806:
610:
377:
372:
367:
362:
357:
352:
38:
25:
1390:
1100:, the strongest known superacid
880: values down to −28.
692:Case Western Reserve University
606:(at 25 °C , 100 kPa).
1362:
1335:
1308:
1200:
1173:
1151:
316:Occupational safety and health
1:
1104:
1033:Catalysis with hydroperoxides
776:
1344:Journal of Organic Chemistry
1158:Lesney, M. S. (March 2003).
1125:Journal of Organic Chemistry
781:
7:
1091:
829:
713:even weak bases, including
10:
1466:
732:
296:316.82 g/mol
1082:
709:, and have been shown to
600:
333:
313:
308:
265:
245:
210:
61:
51:
46:
37:
24:
836:Hammett acidity function
456:Precautionary statements
1208:of the Hydronium Ion".
1167:Today's Chemist at Work
883:
257:OS(=O)(=O)F.F(F)(F)(F)F
1252:10.1002/anie.197301731
967:In the presence of FSO
944:Reactions with alkanes
707:saturated hydrocarbons
672:antimony pentafluoride
559:
1440:Antimony(V) compounds
558:
1098:Fluoroantimonic acid
1055:Catalysis with ozone
896:Lewis dot structures
871:Fluoroantimonic acid
541:(fire diamond)
1415:10.1021/ja00433a035
1384:10.1021/ja00439a038
1356:10.1021/jo00399a014
1329:10.1021/ja00424a038
1302:10.1021/ja01012a066
1222:10.1021/ja01039a019
1183:Superacid Chemistry
759:fluorosulfuric acid
739:James Bryant Conant
664:fluorosulfuric acid
510:P305+P351+P338+P310
21:
925:Raman spectroscopy
727:molecular hydrogen
678:). This conjugate
633:Infobox references
560:
19:
1409:(17): 5261–5268.
1378:(23): 7341–7345.
1296:(10): 2726–2727.
1216:(11): 2929–2941.
1193:978-0-471-59668-4
1137:10.1021/jo040285o
971:D rather than FSO
917:nonclassical ions
641:Chemical compound
639:
638:
402:Hazard statements
179:CompTox Dashboard
103:Interactive image
1457:
1450:Fluoro complexes
1419:
1418:
1394:
1388:
1387:
1366:
1360:
1359:
1339:
1333:
1332:
1323:(8): 2245–2250.
1312:
1306:
1305:
1287:
1286:
1285:
1265:
1256:
1255:
1235:
1226:
1225:
1204:
1198:
1197:
1177:
1171:
1170:
1164:
1155:
1149:
1148:
1131:(7): 2413–2429.
1120:
1075:
1046:
1024:
1016:temperatures.
1011:
1002:
1001:
1000:
990:
989:
988:
963:
962:
961:
935:
911:
810:
745:could protonate
700:hypercoordinated
623:
617:
614:
613:
591:
588:
580:
573:
566:
551:
531:
527:
523:
519:
515:
511:
507:
503:
499:
495:
491:
487:
483:
479:
475:
471:
467:
463:
449:
445:
441:
437:
433:
429:
425:
421:
417:
413:
409:
381:
376:
371:
366:
361:
356:
273:Chemical formula
203:
202:
187:
185:
169:
158:
147:
136:
125:
105:
81:
42:
29:
22:
18:
1465:
1464:
1460:
1459:
1458:
1456:
1455:
1454:
1425:
1424:
1423:
1422:
1400:
1395:
1391:
1367:
1363:
1340:
1336:
1313:
1309:
1284:
1281:
1280:
1279:
1277:
1275:
1271:
1266:
1259:
1236:
1229:
1205:
1201:
1194:
1178:
1174:
1162:
1156:
1152:
1121:
1112:
1107:
1094:
1085:
1062:
1057:
1035:
999:
996:
995:
994:
992:
987:
984:
983:
982:
980:
978:
974:
970:
960:
957:
956:
955:
953:
946:
921:IR spectroscopy
891:
886:
879:
868:
864:
860:
855:perchloric acid
852:
845:
841:
832:
825:
801:
797:
793:
789:
784:
779:
755:R. J. Gillespie
743:perchloric acid
735:
677:
669:
656:
652:
642:
635:
630:
629:
628: ?)
619:
615:
611:
607:
596:
595:
594:
593:
589:
586:
582:
575:
568:
561:
557:
549:
458:
404:
390:
349:
326:
285:
281:
275:
261:
258:
253:
252:
241:
238:
237:
234:
228:
227:
224:
218:
217:
206:
188:
181:
172:
159:
128:
108:
95:
84:
71:
57:
33:
30:
17:
12:
11:
5:
1463:
1453:
1452:
1447:
1445:Sulfonic acids
1442:
1437:
1421:
1420:
1398:
1389:
1361:
1350:(5): 865–867.
1334:
1307:
1282:
1273:
1269:
1257:
1246:(3): 173–254.
1227:
1199:
1192:
1172:
1150:
1109:
1108:
1106:
1103:
1102:
1101:
1093:
1090:
1084:
1081:
1077:
1076:
1060:
1056:
1053:
1048:
1047:
1034:
1031:
1026:
1025:
1013:
1012:
997:
985:
976:
972:
968:
958:
945:
942:
937:
936:
913:
912:
890:
887:
885:
882:
877:
866:
862:
858:
850:
843:
839:
831:
828:
823:
812:
811:
799:
795:
791:
787:
783:
780:
778:
775:
734:
731:
703:carbonium ions
675:
667:
654:
650:
640:
637:
636:
631:
609:
608:
604:standard state
601:
598:
597:
583:
576:
569:
562:
547:
546:
545:
544:
542:
533:
532:
506:P304+P340+P310
502:P303+P361+P353
498:P301+P330+P331
459:
454:
451:
450:
405:
400:
397:
396:
391:
386:
383:
382:
350:
345:
342:
341:
331:
330:
327:
324:
321:
320:
311:
310:
306:
305:
302:
298:
297:
294:
288:
287:
283:
279:
276:
271:
268:
267:
263:
262:
260:
259:
256:
248:
247:
246:
243:
242:
240:
239:
235:
232:
231:
229:
225:
222:
221:
213:
212:
211:
208:
207:
205:
204:
196:DTXSID00894108
191:
189:
177:
174:
173:
171:
170:
162:
160:
152:
149:
148:
138:
130:
129:
127:
126:
118:
116:
110:
109:
107:
106:
98:
96:
89:
86:
85:
83:
82:
74:
72:
67:
64:
63:
59:
58:
55:
49:
48:
44:
43:
35:
34:
31:
15:
9:
6:
4:
3:
2:
1462:
1451:
1448:
1446:
1443:
1441:
1438:
1436:
1433:
1432:
1430:
1416:
1412:
1408:
1404:
1393:
1385:
1381:
1377:
1373:
1365:
1357:
1353:
1349:
1345:
1338:
1330:
1326:
1322:
1318:
1311:
1303:
1299:
1295:
1291:
1264:
1262:
1253:
1249:
1245:
1241:
1234:
1232:
1223:
1219:
1215:
1211:
1203:
1195:
1189:
1185:
1184:
1176:
1168:
1161:
1154:
1146:
1142:
1138:
1134:
1130:
1126:
1119:
1117:
1115:
1110:
1099:
1096:
1095:
1089:
1080:
1074:
1070:
1069:
1068:
1066:
1052:
1045:
1041:
1040:
1039:
1030:
1023:
1019:
1018:
1017:
1010:
1006:
1005:
1004:
965:
951:
941:
934:
930:
929:
928:
926:
922:
918:
910:
906:
905:
904:
902:
897:
881:
876:
872:
856:
849:
837:
827:
819:
817:
809:
805:
804:
803:
774:
772:
768:
764:
760:
756:
752:
748:
744:
740:
730:
728:
724:
720:
716:
712:
708:
704:
701:
697:
693:
689:
685:
681:
673:
665:
661:
657:
646:
634:
627:
622:
605:
599:
592:
581:
574:
567:
543:
540:
539:
535:
534:
460:
457:
453:
452:
406:
403:
399:
398:
395:
392:
389:
385:
384:
380:
375:
370:
365:
360:
355:
351:
348:
344:
343:
339:
337:
332:
328:
323:
322:
318:
317:
312:
307:
303:
300:
299:
295:
293:
290:
289:
277:
274:
270:
269:
264:
255:
254:
251:
244:
230:
220:
219:
216:
209:
201:
197:
193:
192:
190:
180:
176:
175:
168:
164:
163:
161:
155:
151:
150:
146:
142:
139:
137:
135:ECHA InfoCard
132:
131:
124:
120:
119:
117:
115:
112:
111:
104:
100:
99:
97:
93:
88:
87:
80:
76:
75:
73:
70:
66:
65:
60:
54:
50:
45:
41:
36:
28:
23:
1406:
1402:
1392:
1375:
1371:
1364:
1347:
1343:
1337:
1320:
1316:
1310:
1293:
1289:
1243:
1239:
1213:
1209:
1202:
1182:
1175:
1166:
1153:
1128:
1124:
1086:
1078:
1058:
1049:
1036:
1027:
1014:
966:
947:
938:
914:
892:
874:
847:
833:
820:
813:
785:
770:
736:
696:carbocations
648:
644:
643:
537:
393:
335:
325:Main hazards
314:
62:Identifiers
901:cyclohexane
763:George Olah
741:found that
688:George Olah
388:Signal word
319:(OHS/OSH):
301:Appearance
266:Properties
141:100.041.727
20:Magic acid
1435:Superacids
1429:Categories
1105:References
869:, is −23.
853:, of −12,
777:Properties
645:Magic acid
347:Pictograms
292:Molar mass
114:ChemSpider
90:3D model (
79:23854-38-8
69:CAS Number
53:IUPAC name
790:F and SbF
782:Structure
751:aldehydes
711:protonate
660:superacid
522:P403+P233
494:P301+P310
338:labelling
1169:: 47–48.
1145:15787527
1092:See also
830:Strength
767:paraffin
723:halogens
680:Brønsted
538:NFPA 704
309:Hazards
167:16211378
123:17339394
950:alkanes
747:ketones
733:History
715:methane
690:lab at
670:F) and
658:) is a
626:what is
624: (
304:Liquid
286:
154:PubChem
1190:
1143:
1083:Safety
857:, HClO
816:formal
725:, and
621:verify
618:
394:Danger
250:SMILES
47:Names
1272:H-SbF
1163:(PDF)
1065:ozone
865:F·SbF
719:xenon
684:Lewis
653:H·SbF
215:InChI
92:JSmol
1401:H".
1188:ISBN
1141:PMID
884:Uses
798::HSO
771:tert
749:and
698:and
674:(SbF
666:(HSO
530:P501
526:P405
518:P390
514:P363
490:P284
486:P280
482:P273
478:P271
474:P270
470:P264
466:P261
462:P234
448:H441
444:H411
440:H410
436:H402
432:H350
428:H335
424:H314
420:H302
416:H290
412:H271
408:H240
278:HSbF
1411:doi
1380:doi
1352:doi
1325:doi
1298:doi
1248:doi
1218:doi
1133:doi
822:SbF
649:FSO
336:GHS
184:EPA
157:CID
1431::
1407:98
1405:.
1376:98
1374:.
1348:43
1346:.
1321:98
1319:.
1294:90
1292:.
1278:CH
1260:^
1244:12
1242:.
1230:^
1214:91
1212:.
1165:.
1139:.
1129:70
1127:.
1113:^
993:CH
981:CH
954:CH
923:,
842:SO
729:.
721:,
717:,
590:OX
528:,
524:,
520:,
516:,
512:,
508:,
504:,
500:,
496:,
492:,
488:,
484:,
480:,
476:,
472:,
468:,
464:,
446:,
442:,
438:,
434:,
430:,
426:,
422:,
418:,
414:,
410:,
340::
282:SO
1417:.
1413::
1399:3
1386:.
1382::
1358:.
1354::
1331:.
1327::
1304:.
1300::
1283:5
1274:5
1270:3
1254:.
1250::
1224:.
1220::
1196:.
1147:.
1135::
1061:2
998:3
986:5
977:2
973:3
969:3
959:5
878:0
875:H
867:5
863:3
859:4
851:0
848:H
844:4
840:2
824:5
800:3
796:5
792:5
788:3
682:–
676:5
668:3
655:5
651:3
647:(
616:N
587:W
579:4
572:0
565:4
284:3
280:6
186:)
182:(
94:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.