464:. The battery can be mounted in any position, since the valves only operate on over-pressure faults. Since the battery system is designed to be recombinant and eliminate the emission of gases on overcharge, room ventilation requirements are reduced, and no acid fume is emitted during normal operation. Flooded cell gas emissions are of little consequence in all but the smallest confined areas, and pose very little threat to a domestic user, so a wet cell battery designed for longevity gives lower costs per kWh. In a gel battery, the volume of free electrolyte that could be released on damage to the case or venting is very small. There is no need (or ability) to check the level of electrolyte or to top up water lost due to electrolysis, thus reducing inspection and maintenance requirements. Wet-cell batteries can be maintained by a self-watering system or by topping up every three months. The requirement to add distilled water is normally caused by overcharging. A well-regulated system should not require top-up more often than every three months.
123:. The cyclon is a spiral wound cell with thin lead foil electrodes. A number of manufacturers seized on the technology to implement it in cells with conventional flat plates. In the mid 1980s, two UK companies, Chloride and Tungstone, simultaneously introduced ten year life AGM batteries in capacities up to 400 Ah, stimulated by a British Telecom specification for batteries for support of new digital exchanges. In the same period, Gates acquired another UK company, Varley, specialising in aircraft and military batteries. Varley adapted the Cyclon lead foil technology to produce flat plate batteries with exceptional high rate output. These gained approval for a variety of aircraft including the BAE 125 and 146 business jets, the Harrier and its derivative the AV8B, and some F16 variants as the first alternatives to then standard
142:
173:
to have water (or electrolyte) added from time to time. In contrast, VRLA batteries retain generated gases within the battery as long as the pressure remains within safe levels. Under normal operating conditions the gases can then recombine within the battery itself, sometimes with the help of a catalyst, and no additional electrolyte is needed. However, if the pressure exceeds safety limits, safety valves open to allow the excess gases to escape, and in doing so regulate the pressure back to safe levels (hence "valve regulated" in "VRLA").
355:(ATVs) on the market use AGM batteries to reduce likelihood of acid spilling during cornering, vibration, or after accidents, and for packaging reasons. The lighter, smaller battery can be installed at an odd angle if needed for the design of the motorcycle. Due to the higher manufacturing costs compared with flooded lead-acid batteries, AGM batteries are currently used on luxury vehicles. As vehicles become heavier and equipped with more electronic devices such as navigation and
293:
20:
1463:
240:
435:
AGM and gel-cell batteries are also used for recreational marine purposes, with AGM being more commonly available. AGM deep-cycle marine batteries are offered by a number of suppliers. They typically are favored for their low maintenance and spill-proof quality, although generally considered a less
204:
are woven into a mat to increase the surface area enough to hold a sufficient amount of electrolyte on the cells for their lifetime. The fibers that compose the fine glass mat do not absorb and are not affected by the acidic electrolyte. These mats are wrung out 2–5% after being soaked in acids just
304:
was produced in the early 1930s for portable valve (tube) radio LT supply (2, 4 or 6 V) by adding silica to the sulfuric acid. By this time the glass case was being replaced by celluloid and later in 1930s other plastics. Earlier "wet" cells in glass jars used special valves to allow tilt from
172:
will occur, decomposing water into hydrogen and oxygen, in addition to the intended conversion of lead sulfate and water into lead dioxide, lead, and sulfuric acid (the reverse of the discharge process). If these gases are allowed to escape, as in a conventional flooded cell, the battery will need
475:
charging process: bulk charge, absorption charge, and (maintenance) float charge stages. All lead-acid batteries, irrespective of type, are quick to bulk charge to about 70% of capacity during which the battery will accept a large current input, determined at a voltage setpoint, within a few hours
451:
are recommended for deployment in the
Outside Plant (OSP) at locations such as Controlled Environmental Vaults (CEVs), Electronic Equipment Enclosures (EEEs), and huts, and in uncontrolled structures such as cabinets. Relative to VRLA in telecommunications, the use of VRLA Ohmic Measurement Type
167:
When a cell discharges, the lead and diluted acid undergo a chemical reaction that produces lead sulfate and water. When a cell is subsequently charged, the lead sulfate and water are turned back into lead and acid. In all lead-acid battery designs, charging current must be adjusted to match the
188:. They are not permanently sealed, but are designated to be maintenance free. They can be oriented in any manner, unlike normal lead-acid batteries, which must be kept upright to avoid acid spills and to keep the plates' orientation vertical. Cells may be operated with the plates horizontal (
94:
Due to their construction, the gel cell and AGM types of VRLA can be mounted in any orientation, and do not require constant maintenance. The term "maintenance free" is a misnomer as VRLA batteries still require cleaning and regular functional testing. They are widely used in large portable
452:
Equipment (OMTE) and OMTE-like measurement equipment is a fairly new process to evaluate telecommunications battery plants. The proper use of ohmic test equipment allows battery testing without the need to remove batteries from service to perform costly and time-consuming discharge tests.
164:. VRLA cells have the same chemistry, except the electrolyte is immobilized. In AGM this is accomplished with a fiberglass mat; in gel batteries or "gel cells", the electrolyte is in the form of a paste like gel created by adding silica and other gelling agents to the electrolyte.
428:
VRLA batteries are also the standard power source in sailplanes, due to their ability to withstand a variety of flight attitudes and a relatively large ambient temperature range with no adverse effects. However, charging regimes must be adapted with varying temperature.
502:
If the charger fails to supply a sufficient absorption stage charge duration and C-rate (it 'plateaus' or times out, a common fault of cheap solar chargers), and a suitable float charge profile, the battery's capacity and longevity will be dramatically reduced.
524:
Because of calcium added to its plates to reduce water loss, a sealed AGM or gel battery recharges more quickly than a flooded lead-acid battery of either VRLA or conventional design. Compared to flooded batteries, VRLA batteries are more vulnerable to
520:
Lead-acid battery lifetime cycles will vary with the care given, with best care they may achieve 500 to 1000 cycles. With less careful use, a lifetime as few as 100 cycles might be expected (all dependent upon the use environment too).
62:
characterized by a limited amount of electrolyte ("starved" electrolyte) absorbed in a plate separator or formed into a gel; proportioning of the negative and positive plates so that oxygen recombination is facilitated within the
327:, which makes the resulting mass gel like and immobile. Unlike a flooded wet cell lead-acid battery, these batteries do not need to be kept upright. Gel batteries reduce the electrolyte evaporation, spillage (and subsequent
90:
between the battery plates which serves to contain the electrolyte and separate the plates. Both types of VRLA batteries offer advantages and disadvantages compared to flooded vented lead-acid (VLA) batteries or each other.
376:
may use AGM batteries due to their vibration resistance. AGM batteries are also commonly used in classic vehicles since they are much less likely to leak electrolyte, which could damage hard to replace body panels.
676:
208:
The plates in an AGM battery may be of any shape. Some are flat, whereas others are bent or rolled. Both deep cycle and starting type of AGM batteries, are built into a rectangular case according to
421:
VRLA batteries are used extensively in power wheelchairs and mobility scooters, as the extremely low gas and acid output makes them much safer for indoor use. VRLA batteries are also used in the
1280:
Vinal, G.W. (1955 Jan 01) Storage batteries. A general treatise on the physics and chemistry of secondary batteries and their engineering applications. Energy
Citations Database (ECD) :
491:
rate gradually reduces, and the battery will not accept a higher C-rate. When the absorption stage voltage setpoint is reached (and charge current has tapered off), the charger switches to a
1097:"Exide Earns First-Ever Production Contract Awarded by U.S. Navy for Valve-Regulated Submarine Batteries; Shift to Advanced Product Prompts Closure of Kankakee, Illinois, Battery Plant"
181:
Each cell in a VRLA battery has a pressure relief valve which will activate when the battery starts building pressure of hydrogen gas, generally a result of being recharged.
700:
669:
400:
487:
However, they then require a longer time spent in the current-tapering off intermediate absorption charge stage after the initial bulk charge, when the LA battery
648:
532:
AGM automobile batteries are typically about twice the price of flooded-cell batteries in a given BCI size group; gel batteries as much as five times the price.
218:
As with lead-acid batteries, in order to maximize the life of an AGM battery, it is important to follow the manufacturer's charging specifications. The use of a
1306:
1190:(stating sealed battery plates are hardened with calcium to reduce water loss which "raises the batteries' internal resistance and prevents rapid charging.")
200:
AGM batteries differ from flooded lead-acid batteries in that the electrolyte is held in the glass mats, as opposed to freely flooding the plates. Very thin
488:
432:
VRLA batteries are used in the US Nuclear
Submarine fleet, due to their power density, elimination of gassing, reduced maintenance, and enhanced safety.
111:
The first lead-acid gel battery was invented by
Elektrotechnische Fabrik Sonneberg in 1934. The modern gel or VRLA battery was invented by Otto Jache of
1310:
1096:
359:, AGM batteries are being employed to lower vehicle weight and provide better electrical reliability compared with flooded lead-acid batteries.
510:, and kept at a full charge level by a float source when stored or idle (or stored dry new from the factory, an uncommon practice today).
184:
The cell covers typically have gas diffusers built into them that allow safe dispersal of any excess hydrogen that may be formed during
495:
setpoint at a very low C-rate to maintain the battery's fully charged state indefinitely (the float stage offsets the battery's normal
948:
529:
during abusive charging. The electrolyte cannot be tested by hydrometer to diagnose improper charging that can reduce battery life.
1392:
1348:– Method of making a lead storage battery and lead storage battery made according to this method. Otto Jache's and Heinz Schroeder
513:
When working a discharge cycle, a LA battery should be kept at a DOD of less than 50%, ideally no more than 20-40% DOD; a true LA
99:
systems and similar roles, where large amounts of storage are needed at a lower cost than other low-maintenance technologies like
1121:
444:
1012:
1250:
1204:
1183:
506:
To ensure maximum life, a lead-acid battery should be fully recharged as soon after a discharge cycle as possible to prevent
86:). Gel cells add silica dust to the electrolyte, forming a thick putty-like gel. AGM (absorbent glass mat) batteries feature
711:
1663:
1550:
748:
607:
449:
Valve-Regulated Lead–Acid (VRLA) Battery String
Certification Levels Based on Requirements for Safety and Performance,
215:
AGM batteries are more resistant to self discharging than conventional batteries within a wide range of temperatures.
1143:
924:
836:
632:
279:
517:
can be taken to a lower DOD (even an occasional 80%), but these greater DOD cycles always impose a longevity price.
1703:
1878:
1713:
1358:
372:
and computer control to ensure the alternator charges the battery when the car is decelerating. Vehicles used in
67:; and the presence of a relief valve that retains the battery contents independent of the position of the cells.
1641:
987:
905:"VRLA battery capacity cycling: Influences of physical design, materials, and methods to evaluate their effect"
890:
573:
261:
1723:
257:
209:
1274:
Valve-Regulated Lead-Acid
Batteries. Edited by Patrick T. Moseley, Jurgen Garche, C.D. Parker, D.A.J. Rand.
1124:, Generic Requirements for Valve-Regulated Lead–Acid (VRLA) Battery Ohmic Measurement Type Equipment (OMTE).
1038:
418:. AGM batteries, due to their lack of free electrolyte, will not crack and leak in these cold environments.
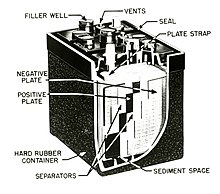
1385:
422:
356:
24:
1281:
460:
VRLA gel and AGM batteries offer several advantages compared with VRLA flooded lead-acid and conventional
1688:
1555:
368:
from March 2007 incorporate AGM batteries in conjunction with devices for recovering brake energy using
226:(DOD) and the cycle life of the battery, with differences between 500 and 1300 cycles depending on DOD.
1728:
1698:
1683:
1651:
1063:
124:
141:
1743:
1490:
219:
1758:
1656:
851:
Robert Nelson, "The Basic
Chemistry of Gas Recombination in Lead–Acid Batteries", JOM 53 (1) (2001)
1763:
1708:
1693:
1646:
1626:
1585:
1560:
1540:
1520:
1378:
1364:
792:
John Devitt (1997). "An account of the development of the first valve-regulated lead/acid cell".
467:
An underlying disadvantage with all lead-acid batteries is the requirement for a relatively long
461:
250:
305:
vertical to one horizontal direction in 1927 to 1931 or 1932. The gel cells were less likely to
1883:
1852:
1753:
1239:
764:
440:
411:
185:
1275:
1718:
1614:
1535:
861:
568:
472:
136:
59:
1596:
1580:
1565:
1401:
801:
468:
369:
64:
909:
INTELEC - Twentieth
International Telecommunications Energy Conference (Cat. No.98CH36263)
8:
1748:
1733:
1668:
1636:
1631:
1447:
963:
827:
Wagner, R (2004-03-09). "13.3 Gel batteries". In
Moseley, Patrick T; et al. (eds.).
765:"Handbook for Gel-VRLA-Batteries : Part 1 : Basic Principles, Design, Features"
352:
100:
1160:
805:
1738:
1609:
1420:
930:
514:
223:
146:
1462:
1176:
Managing 12 Volts: How to
Upgrade, Operate and Troubleshoot 12 Volt Electrical Systems
813:
1812:
1500:
1246:
1179:
1139:
934:
920:
886:
832:
744:
628:
603:
396:
116:
1530:
335:. Chemically they are almost the same as wet (non sealed) batteries except that the
331:
problems) common to the wet cell battery, and boast greater resistance to shock and
1678:
1673:
1485:
1425:
1321:
912:
809:
578:
481:
168:
ability of the battery to absorb the energy. If the charging current is too great,
1208:
1545:
1472:
1287:
526:
306:
87:
439:
In telecommunications applications, VRLA batteries that comply with criteria in
1781:
1435:
916:
496:
392:
28:
1344:
1336:
1328:
1316:
1205:"FAQ: What is the Best Battery System to Use for an Auxiliary Charging System"
904:
296:
Broken gel battery with white gobbets of the gelated electrolyte on the plates
1873:
1867:
1415:
1301:
1100:
492:
320:
161:
741:
Innovators in Battery Technology: Profiles of 95 Influential Electrochemists
546:
1575:
1510:
1452:
1430:
381:
362:
324:
169:
96:
292:
112:
1847:
1832:
1570:
1495:
555:
AGM batteries are by nature, safer for the environment, and safer to use.
384:
373:
316:
157:
1370:
1791:
1525:
1505:
388:
264: in this section. Unsourced material may be challenged and removed.
201:
542:
Cannot tolerate overcharging: overcharging leads to premature failure.
1837:
1827:
1817:
1786:
1442:
507:
332:
328:
153:
1359:
Why do I need a special battery for the automatic start-stop system?
1013:"AGM Discharge Characteristics : Modified on: Mon, 6 Oct, 2014"
239:
1515:
959:
336:
19:
1822:
862:"The Basic Chemistry of Gas Recombination in Lead–Acid Batteries"
340:
120:
903:
Vaccaro, F.J.; Rhoades, J.; Le, B.; Malley, R. (October 1998).
477:
436:
cost effective solution relative to traditional flooded cells.
415:
649:"Exploding Lead Acid Batteries, Mines Safety Bulletin No. 150"
410:
AGM batteries are routinely chosen for remote sensors such as
152:
Lead-acid cells consist of two plates of lead, which serve as
1807:
1138:(Eleventh ed.). New York: McGraw-Hill. pp. 11–116.
1005:
1039:"Exide Gel-Cel Accumulator JSK2 Power-S Chloride Electrical"
539:
Have shorter recharge time than flooded lead-acid batteries.
1288:
The Absorption of Sulfur Dioxide by the Gel of Silicic Acid
881:
Ronald Dell, David Anthony James Rand, Robert Bailey, Jr.,
404:
1062:
Walchhofer, Hans Martin; Watterson, Michael (2013-11-27).
545:
Have shorter useful life, compared to properly maintained
455:
222:
is recommended. There is a direct correlation between the
1842:
1340:– Lead acid battery plate with starch coated glass fibers
1086:
Linden, Reddy (ed), Handbook of batteries, third ed, 2002
365:
313:
602:(Sixth ed.). McGraw Hill Professional. p. 48.
115:
in 1957. The first AGM cell was the Cyclon, patented by
902:
476:(with a charge source capable of supplying the design
425:(UPS) as a backup when the electrical power goes off.
1305:– Treatment Of Porous Pots For Electric Batteries.
1064:"Super Range Portable four A (without tuning dial)"
1061:
1245:(2nd ed.). International Marine. p. 11.
1238:
675:. Trojan Battery Company, California, USA. 2018.
1865:
701:"A Brief History of Batteries and Stored Energy"
1198:
1196:
70:There are two primary types of VRLA batteries,
734:
732:
651:. Australia: Queensland Government. 2015-10-27
623:Linden, David B.; Reddy, Thomas (2002). "24".
312:A modern gel battery is a VRLA battery with a
1386:
558:Can be used or positioned in any orientation.
1241:Boatowner's Mechanical and Electrical Manual
1193:
988:"AGM Charging : Technical Support Desk"
195:
23:A 12V VRLA battery, typically used in small
791:
729:
670:"Selecting the Proper Lead–Acid Technology"
622:
309:when the portable set was handled roughly.
1393:
1379:
1320:– Solid Acid Storage Battery Electrolyte.
1136:Standard Handbook for Electrical Engineers
552:Discharge significantly less hydrogen gas.
380:Deep-cycle AGMs are also commonly used in
1400:
1232:
1230:
1228:
1226:
1134:Fink, Donald G.; Beaty, H. Wayne (1978).
1133:
1036:
949:"Technical Manual: Powersports Batteries"
280:Learn how and when to remove this message
1202:
1178:. Summer Breeze Publishing. p. 44.
877:
875:
343:, and gas recombination can take place.
291:
140:
18:
1158:
738:
456:Comparison with flooded lead-acid cells
1866:
1236:
1223:
826:
739:Desmond, Kevin (2016). "Jache, Otto".
597:
192:style), which may improve cycle life.
1374:
1173:
872:
600:Aircraft Electricity and Electronics
471:cycle time arising from an inherent
262:adding citations to reliable sources
233:
1268:
885:,Royal Society of Chemistry, 2001,
829:Valve-Regulated Lead–Acid Batteries
625:Handbook of Batteries Third Edition
212:(BCI) battery code specifications.
13:
1290:. Eschenbach Print. Company, 1920.
1263:
785:
339:in the lead plates is replaced by
130:
14:
1895:
1352:
1037:Watterson, Michael (2014-06-28).
831:. Elsevier Science. p. 446.
1461:
238:
125:nickel–cadmium (Ni-Cd) batteries
1167:
1161:"What is a Deep Cycle Battery?"
1152:
1127:
1115:
1089:
1080:
1055:
1030:
980:
941:
896:
854:
845:
682:from the original on 2023-09-29
346:
249:needs additional citations for
205:prior to finish manufacturing.
176:
1365:Pros and cons of AGM batteries
1332:– Composite battery plate grid
1159:Collins, Rod (April 7, 2015).
820:
757:
693:
662:
641:
616:
591:
229:
25:uninterruptible power supplies
1:
814:10.1016/S0378-7753(96)02516-5
584:
210:Battery Council International
535:AGM and gel VRLA batteries:
423:uninterruptible power supply
351:Many modern motorcycles and
119:in 1972 and now produced by
7:
562:
10:
1900:
1294:
1203:Sterling, Charles (2009).
917:10.1109/INTLEC.1998.793494
598:Eismin, Thomas K. (2013).
134:
106:
1800:
1772:
1594:
1551:Metal–air electrochemical
1470:
1459:
1408:
484:for a given Ah battery).
220:voltage regulated charger
196:Absorbent glass mat (AGM)
36:valve regulated lead-acid
16:Type of lead–acid battery
1017:Support.rollsbattery.com
992:Support.rollsbattery.com
794:Journal of Power Sources
395:bank and in large-scale
117:Gates Rubber Corporation
1367:, published by Lifeline
883:Understanding Batteries
145:Cutaway view of a 1953
1879:Rechargeable batteries
1853:Semipermeable membrane
1642:Lithium–iron–phosphate
1237:Calder, Nigel (1996).
1174:Barre, Harold (1997).
443:requirements document
441:Telcordia Technologies
297:
160:consisting of diluted
149:
46:, commonly known as a
31:
1724:Rechargeable alkaline
1402:Electrochemical cells
1345:U.S. patent 4,414,302
1337:U.S. patent 4,238,557
1329:U.S. patent 4,134,192
1317:U.S. patent 3,271,199
569:List of battery types
300:Originally a kind of
295:
144:
22:
1704:Nickel–metal hydride
1361:, published by Varta
911:. pp. 166–172.
391:installations as an
370:regenerative braking
353:all-terrain vehicles
258:improve this article
95:electrical devices,
1714:Polysulfide–bromide
1556:Nickel oxyhydroxide
1448:Thermogalvanic cell
1307:Erhard Ludwig Mayer
1302:U.S. patent 417,392
806:1997JPS....64..153D
717:on 20 February 2019
462:lead-acid batteries
72:absorbent glass mat
1477:(non-rechargeable)
1421:Concentration cell
956:YuasaBatteries.com
893:p. 101, pp.120-122
515:deep-cycle battery
298:
224:depth of discharge
156:, suspended in an
150:
147:automotive battery
32:
1861:
1860:
1282:Document #7308501
1252:978-0-07-009618-9
1185:978-0-9647386-1-4
489:charge acceptance
357:stability control
290:
289:
282:
137:Lead–acid battery
60:lead-acid battery
1891:
1657:Lithium–titanate
1602:
1478:
1465:
1426:Electric battery
1395:
1388:
1381:
1372:
1371:
1347:
1339:
1331:
1322:Alexander Koenig
1319:
1304:
1269:Books and papers
1257:
1256:
1244:
1234:
1221:
1220:
1218:
1216:
1211:on 16 March 2012
1207:. Archived from
1200:
1191:
1189:
1171:
1165:
1164:
1156:
1150:
1149:
1131:
1125:
1119:
1113:
1112:
1110:
1108:
1093:
1087:
1084:
1078:
1077:
1075:
1074:
1059:
1053:
1052:
1050:
1049:
1034:
1028:
1027:
1025:
1023:
1009:
1003:
1002:
1000:
998:
984:
978:
977:
975:
974:
968:
962:. Archived from
953:
945:
939:
938:
900:
894:
879:
870:
869:
858:
852:
849:
843:
842:
824:
818:
817:
800:(1–2): 153–156.
789:
783:
782:
780:
778:
772:Sonnenschein.org
769:
761:
755:
754:
736:
727:
726:
724:
722:
716:
710:. Archived from
705:
697:
691:
690:
688:
687:
681:
674:
666:
660:
659:
657:
656:
645:
639:
638:
620:
614:
613:
595:
547:wet-cell battery
414:stations in the
397:amateur robotics
285:
278:
274:
271:
265:
242:
234:
48:sealed lead-acid
1899:
1898:
1894:
1893:
1892:
1890:
1889:
1888:
1864:
1863:
1862:
1857:
1796:
1775:
1768:
1689:Nickel–hydrogen
1647:Lithium–polymer
1603:
1600:
1599:
1590:
1479:
1476:
1475:
1466:
1457:
1404:
1399:
1355:
1343:
1335:
1327:
1315:
1300:
1297:
1286:John McGavack.
1271:
1266:
1264:Further reading
1261:
1260:
1253:
1235:
1224:
1214:
1212:
1201:
1194:
1186:
1172:
1168:
1157:
1153:
1146:
1132:
1128:
1120:
1116:
1106:
1104:
1095:
1094:
1090:
1085:
1081:
1072:
1070:
1068:RadioMuseum.org
1060:
1056:
1047:
1045:
1043:RadioMuseum.org
1035:
1031:
1021:
1019:
1011:
1010:
1006:
996:
994:
986:
985:
981:
972:
970:
966:
951:
947:
946:
942:
927:
901:
897:
880:
873:
860:
859:
855:
850:
846:
839:
825:
821:
790:
786:
776:
774:
767:
763:
762:
758:
751:
737:
730:
720:
718:
714:
703:
699:
698:
694:
685:
683:
679:
672:
668:
667:
663:
654:
652:
647:
646:
642:
635:
627:. McGraw-Hill.
621:
617:
610:
596:
592:
587:
565:
527:thermal runaway
458:
349:
286:
275:
269:
266:
255:
243:
232:
198:
179:
139:
133:
131:Basic principle
109:
88:fiberglass mesh
58:, is a type of
29:emergency lamps
17:
12:
11:
5:
1897:
1887:
1886:
1881:
1876:
1859:
1858:
1856:
1855:
1850:
1845:
1840:
1835:
1830:
1825:
1820:
1815:
1810:
1804:
1802:
1798:
1797:
1795:
1794:
1789:
1784:
1782:Atomic battery
1778:
1776:
1773:
1770:
1769:
1767:
1766:
1761:
1756:
1754:Vanadium redox
1751:
1746:
1741:
1736:
1731:
1729:Silver–cadmium
1726:
1721:
1716:
1711:
1706:
1701:
1699:Nickel–lithium
1696:
1691:
1686:
1684:Nickel–cadmium
1681:
1676:
1671:
1666:
1661:
1660:
1659:
1654:
1652:Lithium–sulfur
1649:
1644:
1639:
1629:
1624:
1623:
1622:
1612:
1606:
1604:
1601:(rechargeable)
1597:Secondary cell
1595:
1592:
1591:
1589:
1588:
1583:
1578:
1573:
1568:
1563:
1558:
1553:
1548:
1543:
1538:
1533:
1528:
1523:
1521:Edison–Lalande
1518:
1513:
1508:
1503:
1498:
1493:
1488:
1482:
1480:
1471:
1468:
1467:
1460:
1458:
1456:
1455:
1450:
1445:
1440:
1439:
1438:
1436:Trough battery
1433:
1423:
1418:
1412:
1410:
1406:
1405:
1398:
1397:
1390:
1383:
1375:
1369:
1368:
1362:
1354:
1353:External links
1351:
1350:
1349:
1341:
1333:
1325:
1313:
1311:Henry Liepmann
1296:
1293:
1292:
1291:
1284:
1278:
1270:
1267:
1265:
1262:
1259:
1258:
1251:
1222:
1192:
1184:
1166:
1151:
1144:
1126:
1114:
1088:
1079:
1054:
1029:
1004:
979:
940:
925:
895:
871:
853:
844:
837:
819:
784:
756:
750:978-1476622781
749:
728:
692:
661:
640:
633:
615:
609:978-0071799157
608:
589:
588:
586:
583:
582:
581:
576:
571:
564:
561:
560:
559:
556:
553:
550:
543:
540:
497:self-discharge
457:
454:
412:ice monitoring
407:competitions.
399:, such as the
393:energy storage
348:
345:
323:is mixed with
288:
287:
246:
244:
237:
231:
228:
197:
194:
178:
175:
135:Main article:
132:
129:
108:
105:
97:off-grid power
15:
9:
6:
4:
3:
2:
1896:
1885:
1884:Sulfuric acid
1882:
1880:
1877:
1875:
1872:
1871:
1869:
1854:
1851:
1849:
1846:
1844:
1841:
1839:
1836:
1834:
1831:
1829:
1826:
1824:
1821:
1819:
1816:
1814:
1811:
1809:
1806:
1805:
1803:
1799:
1793:
1790:
1788:
1785:
1783:
1780:
1779:
1777:
1771:
1765:
1762:
1760:
1757:
1755:
1752:
1750:
1747:
1745:
1744:Sodium–sulfur
1742:
1740:
1737:
1735:
1732:
1730:
1727:
1725:
1722:
1720:
1719:Potassium ion
1717:
1715:
1712:
1710:
1707:
1705:
1702:
1700:
1697:
1695:
1692:
1690:
1687:
1685:
1682:
1680:
1677:
1675:
1672:
1670:
1667:
1665:
1662:
1658:
1655:
1653:
1650:
1648:
1645:
1643:
1640:
1638:
1635:
1634:
1633:
1630:
1628:
1625:
1621:
1618:
1617:
1616:
1613:
1611:
1608:
1607:
1605:
1598:
1593:
1587:
1584:
1582:
1579:
1577:
1574:
1572:
1569:
1567:
1564:
1562:
1559:
1557:
1554:
1552:
1549:
1547:
1544:
1542:
1539:
1537:
1536:Lithium metal
1534:
1532:
1529:
1527:
1524:
1522:
1519:
1517:
1514:
1512:
1509:
1507:
1504:
1502:
1499:
1497:
1494:
1492:
1491:Aluminium–air
1489:
1487:
1484:
1483:
1481:
1474:
1469:
1464:
1454:
1451:
1449:
1446:
1444:
1441:
1437:
1434:
1432:
1429:
1428:
1427:
1424:
1422:
1419:
1417:
1416:Galvanic cell
1414:
1413:
1411:
1407:
1403:
1396:
1391:
1389:
1384:
1382:
1377:
1376:
1373:
1366:
1363:
1360:
1357:
1356:
1346:
1342:
1338:
1334:
1330:
1326:
1323:
1318:
1314:
1312:
1308:
1303:
1299:
1298:
1289:
1285:
1283:
1279:
1277:
1273:
1272:
1254:
1248:
1243:
1242:
1233:
1231:
1229:
1227:
1210:
1206:
1199:
1197:
1187:
1181:
1177:
1170:
1162:
1155:
1147:
1145:0-07-020974-X
1141:
1137:
1130:
1123:
1118:
1102:
1101:Business Wire
1098:
1092:
1083:
1069:
1065:
1058:
1044:
1040:
1033:
1018:
1014:
1008:
993:
989:
983:
969:on 2017-07-12
965:
961:
957:
950:
944:
936:
932:
928:
926:0-7803-5069-3
922:
918:
914:
910:
906:
899:
892:
888:
884:
878:
876:
867:
863:
857:
848:
840:
838:9780444507464
834:
830:
823:
815:
811:
807:
803:
799:
795:
788:
773:
766:
760:
752:
746:
743:. McFarland.
742:
735:
733:
713:
709:
708:Netaworld.org
702:
696:
678:
671:
665:
650:
644:
636:
634:0-07-135978-8
630:
626:
619:
611:
605:
601:
594:
590:
580:
579:Peukert's law
577:
575:
572:
570:
567:
566:
557:
554:
551:
548:
544:
541:
538:
537:
536:
533:
530:
528:
522:
518:
516:
511:
509:
504:
500:
498:
494:
493:float voltage
490:
485:
483:
479:
474:
470:
465:
463:
453:
450:
446:
442:
437:
433:
430:
426:
424:
419:
417:
413:
408:
406:
402:
398:
394:
390:
386:
383:
378:
375:
371:
367:
364:
360:
358:
354:
344:
342:
338:
334:
330:
326:
322:
321:sulfuric acid
318:
315:
310:
308:
303:
294:
284:
281:
273:
270:December 2019
263:
259:
253:
252:
247:This section
245:
241:
236:
235:
227:
225:
221:
216:
213:
211:
206:
203:
193:
191:
187:
182:
174:
171:
165:
163:
162:sulfuric acid
159:
155:
148:
143:
138:
128:
126:
122:
118:
114:
104:
102:
98:
92:
89:
85:
81:
77:
73:
68:
66:
61:
57:
53:
49:
45:
41:
37:
30:
26:
21:
1759:Zinc–bromine
1619:
1566:Silver oxide
1501:Chromic acid
1473:Primary cell
1453:Voltaic pile
1431:Flow battery
1240:
1213:. Retrieved
1209:the original
1175:
1169:
1154:
1135:
1129:
1122:GR-3169-CORE
1117:
1105:. Retrieved
1091:
1082:
1071:. Retrieved
1067:
1057:
1046:. Retrieved
1042:
1032:
1020:. Retrieved
1016:
1007:
995:. Retrieved
991:
982:
971:. Retrieved
964:the original
955:
943:
908:
898:
882:
865:
856:
847:
828:
822:
797:
793:
787:
775:. Retrieved
771:
759:
740:
719:. Retrieved
712:the original
707:
695:
684:. Retrieved
664:
653:. Retrieved
643:
624:
618:
599:
593:
574:Sand battery
534:
531:
523:
519:
512:
505:
501:
499:over time).
486:
466:
459:
448:
438:
434:
431:
427:
420:
409:
379:
361:
350:
347:Applications
325:fumed silica
311:
301:
299:
276:
267:
256:Please help
251:verification
248:
217:
214:
207:
202:glass fibers
199:
189:
183:
180:
177:Construction
170:electrolysis
166:
151:
113:Sonnenschein
110:
93:
83:
79:
75:
71:
69:
55:
51:
47:
43:
39:
35:
33:
1848:Salt bridge
1833:Electrolyte
1764:Zinc–cerium
1749:Solid state
1734:Silver–zinc
1709:Nickel–zinc
1694:Nickel–iron
1669:Molten salt
1637:Dual carbon
1632:Lithium ion
1627:Lithium–air
1586:Zinc–carbon
1561:Silicon–air
1541:Lithium–air
1107:7 September
1022:19 February
997:19 February
777:19 February
721:19 February
480:bulk stage
473:three-stage
385:solar power
374:auto racing
317:electrolyte
302:gel battery
230:Gel battery
158:electrolyte
101:lithium ion
84:gel battery
1868:Categories
1801:Cell parts
1792:Solar cell
1774:Other cell
1739:Sodium ion
1610:Automotive
1215:2 February
1073:2021-04-07
1048:2015-03-01
973:2019-12-25
891:0854046054
686:2023-09-29
655:2020-02-17
585:References
389:wind power
186:overcharge
154:electrodes
1838:Half-cell
1828:Electrode
1787:Fuel cell
1664:Metal–air
1615:Lead–acid
1531:Leclanché
1443:Fuel cell
935:108814630
508:sulfation
333:vibration
329:corrosion
1818:Catalyst
1679:Nanowire
1674:Nanopore
1620:gel–VRLA
1581:Zinc–air
1486:Alkaline
960:GS Yuasa
677:Archived
563:See also
469:recharge
382:off-grid
363:5 series
337:antimony
80:gel cell
1823:Cathode
1576:Zamboni
1546:Mercury
1511:Daniell
1295:Patents
866:TMS.org
802:Bibcode
482:current
445:GR-4228
341:calcium
314:gelated
190:pancake
121:EnerSys
107:History
56:battery
44:battery
1813:Binder
1571:Weston
1496:Bunsen
1324:et al.
1249:
1182:
1142:
1103:. 2005
933:
923:
889:
835:
747:
631:
606:
478:C-rate
416:Arctic
319:; the
78:) and
1808:Anode
1526:Grove
1506:Clark
1409:Types
967:(PDF)
952:(PDF)
931:S2CID
768:(PDF)
715:(PDF)
704:(PDF)
680:(PDF)
673:(PDF)
401:FIRST
1874:Lead
1843:Ions
1309:and
1276:p202
1247:ISBN
1217:2012
1180:ISBN
1140:ISBN
1109:2016
1024:2019
999:2019
921:ISBN
887:ISBN
833:ISBN
779:2019
745:ISBN
723:2019
629:ISBN
604:ISBN
405:IGVC
403:and
387:and
366:BMWs
307:leak
65:cell
40:VRLA
27:and
1516:Dry
913:doi
810:doi
260:by
76:AGM
52:SLA
34:A
1870::
1225:^
1195:^
1099:.
1066:.
1041:.
1015:.
990:.
958:.
954:.
929:.
919:.
907:.
874:^
864:.
808:.
798:64
796:.
770:.
731:^
706:.
447:,
127:.
103:.
54:)
42:)
1394:e
1387:t
1380:v
1255:.
1219:.
1188:.
1163:.
1148:.
1111:.
1076:.
1051:.
1026:.
1001:.
976:.
937:.
915::
868:.
841:.
816:.
812::
804::
781:.
753:.
725:.
689:.
658:.
637:.
612:.
549:.
283:)
277:(
272:)
268:(
254:.
82:(
74:(
50:(
38:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.