271:
240:
346:
44:
56:
107:
122:
140:
131:
27:, the 2-aminocarboxylates. Amino acids are prevalent in nature, and all of them function as ligands toward the transition metals. Not included in this article are complexes of the amides (including peptide) and ester derivatives of amino acids. Also excluded are the polyamino acids including the
218:
In addition to the amino acids, peptides and proteins bind metal cofactors through their side chains. For the most part, the α-amino and carboxylate groups are unavailable for binding as they are otherwise engaged in the peptide bond. The situation is more complicated for the N-terminal and
86:
Using kinetically inert metal ions, complexes containing monodentate amino acids have been characterized. These complexes exist in either the N or the O linkage isomers. It can be assumed that such monodentate complexes exist transiently for many kinetically labile metal ions (e.g. Zn).
63:
Most commonly, amino acids coordinate to metal ions as N,O bidentate ligands, utilizing the amino group and the carboxylate. They are "L-X" ligands. A five-membered chelate ring is formed. The chelate ring is only slightly ruffled at the sp-hybridized carbon and nitrogen centers.
202:, which exists both in anhydrous and pentacoordinate geometries. When the metal is square planar, these complexes can exist as cis and trans isomers. The stereochemical possibilities increase when the amino acid ligands are not
155:
Mixing simple metal salts with solutions of amino acids near neutral or elevated pH often affords bis- or tris complexes. For metal ions that prefer octahedral coordination, these complexes often adopt the stoichiometry
724:
Denning, R. G.; Piper, T. S. (1966). "Optical
Activity, Absolute Configuration, and Rearrangement Reactions of Tris Amino Acid Complexes of Cobalt(III) with L-Alanine, L-Leucine, and L-Proline".
615:
Denning, R. G.; Piper, T. S. (1966). "Optical
Activity, Absolute Configuration, and Rearrangement Reactions of Tris Amino Acid Complexes of Cobalt(III) with L-Alanine, L-Leucine, and L-Proline".
538:
Baidya, N.; Ndreu, D.; Olmstead, M. M.; Mascharak, P. K. (1991). "Synthesis, Structure, and
Properties of Potassium bis(L-cysteinato-N,S)nickelate(II) sesquihydrate".
179:. These complexes can exist in facial and meridional isomers, both of which are chiral. The stereochemical possibilities increase when the amino acid ligands are not
364:
Organic compounds featuring two or more 2- and 3-aminocarboxylate groups are ligands of extensive use in nature, industry, and research. Famous examples include
497:
M. Scharwitz, T. van
Almsick, W. S. Sheldrick (2007). "(S-Methylcysteinato)(η-pentamethylcyclopentadienyl)iridium(III) Trifluoromethanesulfonate hemihydrate".
67:
For those amino acids containing coordinating substituents, the resulting complexes are more structurally diverse since these substituents can coordinate.
1291:
1328:
1504:
1178:
832:
565:
524:
483:
1522:
1358:
219:
O-terminal residues where the α-amino and carboxylate groups are unavailable, respectively. Especially important in this regard are histidine (
1527:
1395:
401:
293:
Commonly amino acid complexes are prepared by ligand displacement reactions of metal aquo complexes and the conjugate bases of amino acids:
1414:
751:
Iakovidis, A.; Hadjiliadis, N. (1994). "Complex
Compounds of Platinum(II) and (IV) with Amino Acids, Peptides and Their Derivatives".
1485:
1209:
388:
Severin, K.; Bergs, R.; Beck, W. (1998). "Bioorganometallic
Chemistry-Transition Metal Complexes with α-Amino Acids and Peptides".
206:. Homoleptic complexes are also known where the amino carboxylate is tridentate amino acids. One such complex is Ni(κ-histidinate)
1145:
350:
244:
1350:
1029:
599:
1464:
1315:
1236:
1183:
825:
451:
1201:
1153:
251:
776:"Metal Ions and Metal Complexes as Protective Groups of Amino Acids and Peptides – Reactions at Coordinated Amino Acids"
1476:
1431:
1387:
1371:
1173:
1140:
1130:
1017:
228:
220:
195:, and other amino acids, one obtains four stereoisomers. With cysteine, the amino acid binds through N and thiolate.
1517:
1426:
1283:
1082:
283:
224:
1379:
1342:
1337:
1303:
1094:
1059:
1042:
1034:
970:
910:
176:
1548:
1188:
961:
949:
920:
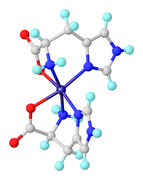
915:
818:
1264:
1168:
1122:
1005:
925:
879:
937:
681:
Motterlini R, Otterbein LE (September 2010). "The therapeutic potential of carbon monoxide". review article.
1456:
1278:
992:
279:
262:) is a spectator ligand. In the area of organometallic complexes, one example of Cp*Ir(κ-methionine).
184:
1440:
1000:
855:
452:"Structural Studies of Bis(histidinato)nickel(II): Combined Experimental and Computational Studies"
1228:
423:
K.-Q. Gu; Y.-X. Sun; R. Zhang; N.-W. Zhang; H.-W. Che (2007). "Tris(glycinato-κN,O)cobalt(III)".
317:, amino acid complexes can be generated by the hydrolysis of amino acid esters and amides (en =
1323:
1135:
314:
642:
Arnold, Alan P.; Jackson, W. Gregory (1990). "Stereospecificity in the
Synthesis of the Tris((
1163:
559:
518:
477:
369:
35:
258:
Mixed ligand complexes are common for amino acids. Well known examples include , where en (
1114:
841:
199:
20:
8:
537:
1509:
1255:
860:
797:
706:
760:
710:
698:
595:
449:
405:
801:
787:
756:
733:
690:
663:
624:
587:
547:
506:
463:
432:
397:
334:
83:
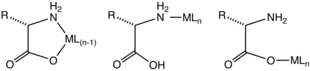
sometimes form tridentate N,N,O, N,O,O, S,N,O, and S,N,O complexes, respectively.
318:
286:. Similar synthetic methods apply to the preparation of tris(chelates) of other
259:
28:
402:
10.1002/(SICI)1521-3773(19980703)37:12<1634::AID-ANIE1634>3.0.CO;2-C
106:
1273:
987:
591:
468:
175:
Complexes of the 3:1 stoichiometry have the formula is . Such complexes adopt
510:
436:
1542:
982:
887:
792:
775:
496:
72:
702:
409:
234:
1047:
810:
737:
667:
628:
551:
287:
203:
180:
76:
24:
68:
59:
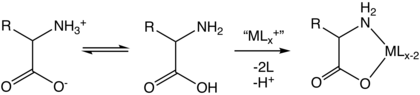
Three coordination modes for 2-aminocarboxylates and related ligands.
694:
422:
139:
121:
270:
130:
80:
239:
192:
188:
150:
43:
337:
for amino acids, allowing diverse reactions of the side chains.
333:
O chelate ring is rather stable, amino acid complexes represent
582:
Kauffman, George B.; Karbassi, Mohammad; Kyuno, Eishin (1989).
450:
A. Abbasi, B. Safarkoopayeh, N. Khosravi, A. Shayesteh (217).
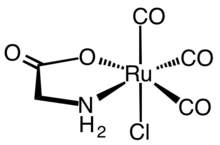
345:
387:
183:. Both the violet meridional and red-pink facial isomers of
365:
31:
55:
198:
Complexes with the 2:1 stoichiometry are illustrated by
586:. Inorganic Syntheses. Vol. 25. pp. 135–139.
235:
Heteroleptic complexes (amino acids plus other ligands)
581:
750:
680:
282:. It is produced by the reaction of glycine with
1540:
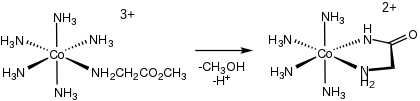
274:Intramolecular route to Co glycinamide complex.
97:Structures of selected complexes of amino acids
654:)-Cysteinesulfinato-N,S)cobaltate(III) Ions".
151:Homoleptic complexes (only amino acid ligands)
90:
826:
773:
641:
340:
160:(aa = amino carboxylate, such as glycinate, H
723:
614:
564:: CS1 maint: multiple names: authors list (
523:: CS1 maint: multiple names: authors list (
482:: CS1 maint: multiple names: authors list (
840:
833:
819:
265:
791:
467:
344:
269:
238:
213:
54:
1541:
577:
575:
23:containing the conjugate bases of the
814:
17:Transition metal amino acid complexes
572:
13:
14:
1560:
284:sodium tris(carbonato)cobalt(III)
187:have been characterized With L-
177:octahedral coordination geometry
138:
129:
120:
105:
50:
42:
767:
744:
717:
753:Coordination Chemistry Reviews
683:Nature Reviews. Drug Discovery
674:
635:
608:
531:
490:
443:
416:
381:
1:
375:
761:10.1016/0010-8545(94)80064-2
329:Because their 5-membered MNC
7:
91:Stoichiometry and structure
10:
1565:
592:10.1002/9780470132562.ch32
584:Tris(glycinato)cobalt(III)
469:10.1016/j.crci.2016.12.006
341:Aminocarboxylate complexes
280:tris(glycinato)cobalt(III)
278:A well studied complex is
185:tris(glycinato)cobalt(III)
1497:
1407:
1248:
1221:
1107:
1075:
903:
872:
848:
511:10.1107/S1600536806053360
437:10.1107/S1600536807005636
793:10.1515/znb-2009-11-1202
250:, known as CORM-3, is a
266:Synthesis and reactions
1549:Coordination chemistry
842:Coordination complexes
774:Wolfgang Beck (2009).
361:
315:bioinorganic chemistry
275:
255:
60:
21:coordination complexes
19:are a large family of
456:Comptes Rendus Chimie
348:
273:
242:
214:Peptides and proteins
58:
390:Angew. Chem. Int. Ed
200:copper(II) glycinate
738:10.1021/ic50040a022
726:Inorganic Chemistry
668:10.1021/ic00343a061
656:Inorganic Chemistry
629:10.1021/ic50040a022
617:Inorganic Chemistry
552:10.1021/ic00010a043
540:Inorganic Chemistry
755:. 135–136: 17–63.
362:
276:
256:
61:
1536:
1535:
662:(18): 3618–3620.
601:978-0-470-13256-2
546:(10): 2448–2451.
396:(12): 1635–1654.
335:protecting groups
252:CO-releasing drug
1556:
835:
828:
821:
812:
811:
806:
805:
795:
771:
765:
764:
748:
742:
741:
732:(6): 1056–1065.
721:
715:
714:
678:
672:
671:
639:
633:
632:
623:(6): 1056–1065.
612:
606:
605:
579:
570:
569:
563:
555:
535:
529:
528:
522:
514:
499:Acta Crystallogr
494:
488:
487:
481:
473:
471:
447:
441:
440:
431:(3): m740–m742.
425:Acta Crystallogr
420:
414:
413:
385:
359:-disuccinic acid
351:ethylenediamine-
300:
142:
133:
124:
109:
46:
29:chelating agents
1564:
1563:
1559:
1558:
1557:
1555:
1554:
1553:
1539:
1538:
1537:
1532:
1513:
1493:
1480:
1472:
1468:
1460:
1452:
1448:
1444:
1435:
1422:
1418:
1403:
1399:
1391:
1383:
1375:
1366:
1362:
1354:
1346:
1332:
1319:
1311:
1307:
1299:
1295:
1287:
1268:
1259:
1244:
1240:
1232:
1217:
1205:
1196:
1192:
1149:
1126:
1118:
1103:
1098:
1090:
1086:
1071:
1067:
1063:
1055:
1051:
1038:
1025:
1021:
1013:
1009:
996:
978:
974:
965:
957:
953:
945:
941:
933:
929:
899:
895:
891:
883:
868:
864:
844:
839:
809:
772:
768:
749:
745:
722:
718:
695:10.1038/nrd3228
679:
675:
640:
636:
613:
609:
602:
580:
573:
557:
556:
536:
532:
516:
515:
495:
491:
475:
474:
448:
444:
421:
417:
386:
382:
378:
343:
332:
319:ethylenediamine
308:
304:
298:
268:
260:ethylenediamine
248:
237:
227:), methionine (
216:
209:
171:
167:
163:
159:
153:
148:
147:
146:
143:
134:
125:
116:
115:
110:
99:
98:
93:
53:
12:
11:
5:
1562:
1552:
1551:
1534:
1533:
1531:
1530:
1525:
1520:
1515:
1511:
1507:
1501:
1499:
1498:Halide donors:
1495:
1494:
1492:
1491:
1483:
1478:
1474:
1470:
1466:
1462:
1458:
1454:
1450:
1446:
1442:
1438:
1433:
1429:
1424:
1420:
1416:
1411:
1409:
1405:
1404:
1402:
1401:
1397:
1393:
1389:
1385:
1381:
1377:
1373:
1369:
1364:
1360:
1356:
1352:
1348:
1344:
1340:
1335:
1330:
1326:
1321:
1317:
1313:
1309:
1305:
1301:
1297:
1293:
1289:
1285:
1281:
1276:
1271:
1266:
1262:
1257:
1252:
1250:
1246:
1245:
1243:
1242:
1238:
1234:
1230:
1225:
1223:
1219:
1218:
1216:
1215:
1207:
1203:
1199:
1194:
1190:
1186:
1181:
1176:
1171:
1166:
1161:
1156:
1151:
1147:
1143:
1138:
1133:
1128:
1124:
1120:
1116:
1111:
1109:
1105:
1104:
1102:
1101:
1096:
1092:
1088:
1084:
1079:
1077:
1073:
1072:
1070:
1069:
1065:
1061:
1057:
1053:
1049:
1045:
1040:
1036:
1032:
1027:
1023:
1019:
1015:
1011:
1007:
1003:
998:
994:
990:
985:
980:
976:
972:
968:
963:
959:
955:
951:
947:
943:
939:
935:
931:
927:
923:
918:
913:
907:
905:
901:
900:
898:
897:
893:
889:
885:
881:
876:
874:
870:
869:
867:
866:
862:
858:
852:
850:
846:
845:
838:
837:
830:
823:
815:
808:
807:
780:Z. Naturforsch
766:
743:
716:
673:
634:
607:
600:
571:
530:
489:
442:
415:
379:
377:
374:
342:
339:
330:
327:
326:
311:
310:
306:
302:
267:
264:
246:
236:
233:
215:
212:
207:
169:
165:
161:
157:
152:
149:
145:
144:
137:
135:
128:
126:
119:
117:
113:
111:
104:
101:
100:
96:
95:
94:
92:
89:
52:
49:
48:
47:
9:
6:
4:
3:
2:
1561:
1550:
1547:
1546:
1544:
1529:
1526:
1524:
1521:
1519:
1516:
1514:
1508:
1506:
1503:
1502:
1500:
1496:
1490:
1489:
1484:
1482:
1475:
1473:
1463:
1461:
1455:
1453:
1439:
1437:
1430:
1428:
1425:
1423:
1413:
1412:
1410:
1406:
1400:
1394:
1392:
1386:
1384:
1378:
1376:
1370:
1368:
1357:
1355:
1349:
1347:
1341:
1339:
1336:
1334:
1327:
1325:
1322:
1320:
1314:
1312:
1302:
1300:
1290:
1288:
1282:
1280:
1277:
1275:
1272:
1270:
1263:
1261:
1254:
1253:
1251:
1247:
1241:
1235:
1233:
1227:
1226:
1224:
1220:
1214:
1212:
1208:
1206:
1200:
1198:
1187:
1185:
1182:
1180:
1177:
1175:
1172:
1170:
1167:
1165:
1162:
1160:
1157:
1155:
1152:
1150:
1144:
1142:
1139:
1137:
1134:
1132:
1129:
1127:
1121:
1119:
1113:
1112:
1110:
1106:
1100:
1093:
1091:
1081:
1080:
1078:
1074:
1068:
1058:
1056:
1046:
1044:
1041:
1039:
1033:
1031:
1028:
1026:
1016:
1014:
1004:
1002:
999:
997:
991:
989:
986:
984:
981:
979:
969:
967:
960:
958:
948:
946:
936:
934:
924:
922:
919:
917:
914:
912:
909:
908:
906:
902:
896:
886:
884:
878:
877:
875:
871:
865:
859:
857:
854:
853:
851:
847:
843:
836:
831:
829:
824:
822:
817:
816:
813:
803:
799:
794:
789:
786:: 1221–1245.
785:
781:
777:
770:
762:
758:
754:
747:
739:
735:
731:
727:
720:
712:
708:
704:
700:
696:
692:
689:(9): 728–43.
688:
684:
677:
669:
665:
661:
657:
653:
650:)- and Tris((
649:
646:)-Cysteinato-
645:
638:
630:
626:
622:
618:
611:
603:
597:
593:
589:
585:
578:
576:
567:
561:
553:
549:
545:
541:
534:
526:
520:
512:
508:
505:: m230-m232.
504:
500:
493:
485:
479:
470:
465:
461:
457:
453:
446:
438:
434:
430:
426:
419:
411:
407:
403:
399:
395:
391:
384:
380:
373:
371:
367:
360:
358:
354:
349:A complex of
347:
338:
336:
324:
323:
322:
320:
316:
296:
295:
294:
291:
289:
285:
281:
272:
263:
261:
253:
249:
245:RuCl(gly)(CO)
243:Structure of
241:
232:
230:
226:
223:), cysteine (
222:
211:
205:
201:
196:
194:
190:
186:
182:
178:
173:
141:
136:
132:
127:
123:
118:
112:Co(glycinate)
108:
103:
102:
88:
84:
82:
78:
74:
73:aspartic acid
70:
65:
57:
51:Binding modes
45:
41:
40:
39:
37:
33:
30:
26:
22:
18:
1487:
1210:
1158:
783:
779:
769:
752:
746:
729:
725:
719:
686:
682:
676:
659:
655:
651:
647:
643:
637:
620:
616:
610:
583:
560:cite journal
543:
539:
533:
519:cite journal
502:
498:
492:
478:cite journal
459:
455:
445:
428:
424:
418:
393:
389:
383:
363:
356:
352:
328:
325:→ + EtOH
313:Relevant to
312:
309:→ + 4 Cl
292:
277:
257:
217:
197:
174:
154:
85:
66:
62:
16:
15:
288:amino acids
25:amino acids
1159:amino acid
1076:Si donors:
462:(5): 467.
376:References
204:homochiral
181:homochiral
77:methionine
1408:S donors:
1249:O donors:
1222:P donors:
1184:porphyrin
1131:imidazole
1108:N donors:
904:C donors:
873:B donors:
849:H donors:
711:205477130
229:thioether
221:imidazole
69:Histidine
1543:Category
802:96555456
703:20811383
410:29711516
305:NCH(R)CO
225:thiolate
81:cysteine
1022:& C
193:leucine
189:alanine
930:=CH-CH
800:
709:
701:
598:
408:
299:
79:, and
921:HC(O)
916:RC(O)
798:S2CID
707:S2CID
156:M(aa)
1324:acac
1296:/HCO
1179:bipy
938:C(CH
699:PMID
596:ISBN
566:link
525:link
484:link
406:PMID
368:and
366:EDTA
297:+ 2
290:.
191:, L-
34:and
32:EDTA
1419:NCS
1396:OPR
1372:OSR
1351:ClO
1338:ONO
1316:RCO
1193:Si)
1189:(Me
1174:RCN
1141:RNO
1089:4−n
1087:SiR
1043:≡CR
1035:=CR
1030:RNC
954:=CH
788:doi
784:64b
757:doi
734:doi
691:doi
664:doi
648:N,S
625:doi
588:doi
548:doi
507:doi
503:E63
464:doi
433:doi
429:E63
398:doi
370:NTA
321:):
231:).
172:).
164:NCH
38:.
36:NTA
1545::
1523:Br
1518:Cl
1486:NC
1477:SR
1457:SO
1427:RS
1388:PO
1380:SO
1367:NO
1343:NO
1333:CO
1292:CO
1274:RO
1237:PR
1229:PR
1213:CS
1169:RN
1154:py
1146:NO
1136:NO
1115:NH
1099:Si
1024:70
1020:60
993:CO
988:CO
983:CN
962:RC
950:CH
926:CH
880:BR
796:.
782:.
778:.
728:.
705:.
697:.
685:.
660:29
658:.
619:.
594:.
574:^
562:}}
558:{{
544:30
542:.
521:}}
517:{{
501:.
480:}}
476:{{
460:20
458:.
454:.
427:.
404:.
394:37
392:.
372:.
357:N'
210:.
168:CO
75:,
71:,
1528:I
1512:2
1510:F
1505:F
1488:S
1481:O
1479:2
1471:3
1469:O
1467:2
1465:S
1459:2
1451:2
1449:S
1447:2
1445:C
1443:2
1441:R
1436:S
1434:2
1432:R
1421:2
1417:2
1415:R
1398:3
1390:4
1382:4
1374:2
1365:5
1363:H
1361:5
1359:C
1353:4
1345:3
1331:2
1329:R
1318:2
1310:4
1308:O
1306:2
1304:C
1298:3
1294:3
1286:2
1284:O
1279:O
1269:O
1267:2
1265:R
1260:O
1258:2
1256:H
1239:2
1231:3
1211:N
1204:2
1202:N
1197:N
1195:2
1191:3
1164:N
1148:2
1125:3
1123:N
1117:3
1097:3
1095:R
1085:n
1083:H
1066:7
1064:H
1062:9
1060:C
1054:5
1052:H
1050:5
1048:C
1037:2
1018:C
1012:6
1010:R
1008:6
1006:C
1001:C
995:2
977:4
975:H
973:6
971:C
966:R
964:2
956:2
952:2
944:3
942:)
940:2
932:2
928:2
911:R
894:n
892:H
890:m
888:B
882:2
863:2
861:H
856:H
834:e
827:t
820:v
804:.
790::
763:.
759::
740:.
736::
730:5
713:.
693::
687:9
670:.
666::
652:R
644:R
631:.
627::
621:5
604:.
590::
568:)
554:.
550::
527:)
513:.
509::
486:)
472:.
466::
439:.
435::
412:.
400::
355:,
353:N
331:2
307:2
303:2
301:H
254:.
247:3
208:2
170:2
166:2
162:2
158:3
114:3
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.