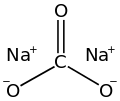
373:
233:
54:
63:
1044:
45:
1107:
2052:
1260:
1110:
1112:
2669:
of ammonium and sodium chlorides; also, more ammonia is pumped at 30-40 °C to this solution. The solution temperature is then lowered to below 10 °C. Solubility of ammonium chloride is higher than that of sodium chloride at 30 °C and lower at 10 °C. Due to this temperature-dependent solubility difference and the
2668:
The sodium bicarbonate was collected as a precipitate due to its low solubility and then heated up to approximately 80 °C (176 °F) or 95 °C (203 °F) to yield pure sodium carbonate similar to last step of the Solvay process. More sodium chloride is added to the remaining solution
1431:
The decahydrate is formed from water solutions crystallizing in the temperature range −2.1 to +32.0 °C, the heptahydrate in the narrow range 32.0 to 35.4 °C and above this temperature the monohydrate forms. In dry air the decahydrate and heptahydrate lose water to give the monohydrate.
1887:
with fiber-reactive dyes, sodium carbonate (often under a name such as soda ash fixative or soda ash activator) is used to ensure proper chemical bonding of the dye with cellulose (plant) fibers, typically before dyeing (for tie dyes), mixed with the dye (for dye painting), or after dyeing (for
1326:. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils, and because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce
2523:). The method is appealing to such users because sodium bicarbonate is widely sold as baking soda, and the temperatures required (250 °F (121 °C) to 300 °F (149 °C)) to convert baking soda to sodium carbonate are readily achieved in conventional kitchen
1682:)). When these materials are heated, the carbonates release carbon dioxide. In this way, sodium carbonate is a source of sodium oxide. Soda–lime glass has been the most common form of glass for centuries. It is also a key input for tableware glass manufacturing.
2078:, Tanzania's unique volcano, and it is presumed to have erupted from other volcanoes in the past, but due to these minerals' instability at the Earth's surface, are likely to be eroded. All three mineralogical forms of sodium carbonate, as well as
2164:" (salt-tolerant) plant species and seaweed species can be processed to yield an impure form of sodium carbonate, and these sources predominated in Europe and elsewhere until the early 19th century. The land plants (typically
2512:. The Solvay process quickly came to dominate sodium carbonate production worldwide. By 1900, 90% of sodium carbonate was produced by the Solvay process, and the last Leblanc process plant closed in the early 1920s.
2152:, have made mining more economical than industrial production in North America. There are important reserves of trona in Turkey; two million tons of soda ash have been extracted from the reserves near Ankara.
1806:
traditionally treated with lye to improve browning can be treated instead with sodium carbonate; sodium carbonate does not produce quite as strong a browning as lye, but is much safer and easier to work with.
1052:
1423:
anhydrous sodium carbonate (natrite), also known as calcined soda, is formed by heating the hydrates. It is also formed when sodium hydrogencarbonate is heated (calcined) e.g. in the final step of the
1576:
2020:
Sodium carbonate is used by the brick industry as a wetting agent to reduce the amount of water needed to extrude the clay. In casting, it is referred to as "bonding agent" and is used to allow wet
3655:
1848:
While it is less likely to cause chemical burns than lye, care must still be taken when working with sodium carbonate in the kitchen, as it is corrosive to aluminum cookware, utensils, and foil.
1024:
2182:" (washed with water) to form an alkali solution. This solution was boiled dry to create the final product, which was termed "soda ash"; this very old name derives from the Arabic word
2515:
The second step of the Solvay process, heating sodium bicarbonate, is used on a small scale by home cooks and in restaurants to make sodium carbonate for culinary purposes (including
3079:"ChemIDplus - 497-19-8 - CDBYLPFSWZWCQE-UHFFFAOYSA-L - Sodium carbonate [NF] - Similar structures search, synonyms, formulas, resource links, and other chemical information"
1111:
2223:", became increasingly inadequate by the end of the 18th century, and the search for commercially viable routes to synthesizing soda ash from salt and other chemicals intensified.
1666:, melting point 1,713 °C), lowering the melting point of the mixture to something achievable without special materials. This "soda glass" is mildly water-soluble, so some
2728:
Harper, J. P. (1936). Antipov, Evgeny; Bismayer, Ulrich; Huppertz, Hubert; Petrícek, Václav; Pöttgen, Rainer; Schmahl, Wolfgang; Tiekink, E. R. T.; Zou, Xiaodong (eds.).
4899:
4565:
4533:
1086:
4629:
1113:
4493:
4252:
4173:
4003:
2101:, and these are expected to include sodium carbonate, deposits have yet to be confirmed, this absence is explained by some as being due to a global dominance of low
4613:
4581:
4549:
4509:
4332:
3995:
2148:
O), is mined in several areas of the US and provides nearly all the US consumption of sodium carbonate. Large natural deposits found in 1938, such as the one near
2692:
by eliminating the production of calcium chloride, since ammonia no longer needs to be regenerated. The by-product ammonium chloride can be sold as a fertilizer.
4653:
4597:
4292:
4043:
1121:
2024:
to adhere to gelled alginate. Sodium carbonate is used in toothpastes, where it acts as a foaming agent and an abrasive, and to temporarily increase mouth pH.
6047:
4067:
5818:
2370:. The soda ash is extracted from the black ash with water. Evaporation of this extract yields solid sodium carbonate. This extraction process was termed
1179:
6419:
5676:
5650:
1273:
3473:
6432:
1135:
1699:
Sodium carbonate is a water-soluble source of carbonate. The calcium and magnesium ions form insoluble solid precipitates upon treatment with
1461:
1696:
Hard water usually contains calcium or magnesium ions. Sodium carbonate is used for removing these ions and replacing them with sodium ions.
3645:
2385:
byproduct also presented waste disposal issues. However, it remained the major production method for sodium carbonate until the late 1880s.
1920:
is itself an intermediate product of the Solvay process, the heating needed to remove the ammonia that contaminates it decomposes some NaHCO
6332:
422:
3348:
5774:
3120:
4741:
2504:
is its only waste product. The process is substantially more economical than the
Leblanc process, which generates two waste products,
1674:" with transition temperature ~570 °C) is made by melting such mixtures of sodium carbonate, calcium carbonate, and silica sand (
150:
2192:, one of the many species of seashore plants harvested for production. "Barilla" is a commercial term applied to an impure form of
1814:
powder. The cooling and fizzing sensation results from the endothermic reaction between sodium carbonate and a weak acid, commonly
1596:
As a cleansing agent for domestic purposes like washing clothes. Sodium carbonate is a component of many dry soap powders. It has
3143:"On the transition temperatures of the transition temperatures of the hydrates of sodium carbonate as fix points in thermometry"
2009:
Sodium carbonate is used by the cotton industry to neutralize the sulfuric acid needed for acid delinting of fuzzy cottonseed.
3448:
3272:
3237:
2927:
2817:
2800:
943:
1268:
3686:
3386:
De
Sanctis, M. C.; et al. (29 June 2016). "Bright carbonate deposits as evidence of aqueous alteration on (1) Ceres".
2449:
The resulting sodium bicarbonate was then converted to sodium carbonate by heating it, releasing water and carbon dioxide:
3092:
974:
3619:
3592:
3216:
3535:
387:
6103:
2959:
2203:
The sodium carbonate concentration in soda ash varied very widely, from 2–3 percent for the seaweed-derived form ("
2074:
The anhydrous mineral form of sodium carbonate is quite rare and called nitrite. Sodium carbonate also erupts from
1916:) or baking soda, also a component in fire extinguishers, is often generated from sodium carbonate. Although NaHCO
1280:
6512:
6120:
2701:
1432:
Other hydrates have been reported, e.g. with 2.5 units of water per sodium carbonate unit ("Penta hemihydrate").
44:
4734:
2067:
have been mined from dry lake bottoms in Egypt since ancient times, when natron was used in the preparation of
2059:
Sodium carbonate is soluble in water, and can occur naturally in arid regions, especially in mineral deposits (
2040:
1005:
53:
5887:
5855:
2006:
This application has become more common, especially where stations have to meet stringent emission controls.
1860:
in various fields. As a common alkali, it is preferred in many chemical processes because it is cheaper than
3267:. International series in the physical and chemical engineering sciences (Ninth ed.). Boston: Pearson.
2012:
It is also used to form carbonates of other metals by ion exchange, often with the other metals' sulphates.
6507:
3549:
1080:
314:
228:
2474:
Meanwhile, the ammonia was regenerated from the ammonium chloride byproduct by treating it with the lime (
1871:
regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic
1452:
O), also known as washing soda, is the most common hydrate of sodium carbonate containing 10 molecules of
6522:
6136:
5530:
5310:
4458:
351:
190:
3372:
5899:
5498:
5451:
5439:
2117:
The initial large-scale chemical procedure was established in
England in 1823 to manufacture soda ash.
1756:
occurs. To take advantage of the former effect, sodium carbonate is therefore one of the components of
1043:
712:
1732:
Sodium carbonate has several uses in cuisine, largely because it is a stronger base than baking soda (
6517:
5550:
5540:
5379:
4727:
3498:
240:
2951:
2093:
Extra terrestrially, known sodium carbonate is rare. Deposits have been identified as the source of
6407:
5522:
5482:
4474:
3679:
1453:
368:
17:
3322:
2889:
2847:
1752:
production in kneaded doughs, and also improves browning by reducing the temperature at which the
5572:
4517:
4019:
3584:
1889:
1872:
731:
5395:
5335:
5322:
4086:
3855:
2129:
1724:
The water is softened because it no longer contains dissolved calcium ions and magnesium ions.
1238:
915:
3438:
3064:
1864:
and far safer to handle. Its mildness especially recommends its use in domestic applications.
6527:
6091:
5722:
4442:
4300:
3956:
3879:
2300:
2094:
1066:
1036:
875:
75:
6364:
6323:
6079:
6007:
5927:
5730:
5605:
5298:
5286:
5135:
4885:
4320:
4079:
4059:
4035:
3802:
3664:
3395:
3295:
3030:
2968:
2637:
2625:
2371:
2179:
2149:
2036:
338:
330:
323:
210:
125:
117:
8:
6502:
6059:
6035:
6019:
5955:
5738:
5696:
5655:
5621:
5363:
5351:
4978:
4871:
4426:
4233:
4142:
3972:
3944:
3891:
3871:
3754:
3746:
3672:
3640:
2805:
1745:
1671:
1343:
1242:
1218:
109:
99:
3399:
3034:
2972:
2837:
1330:), sodium carbonate became known as "soda ash". It is produced in large quantities from
372:
232:
170:
6348:
6311:
5717:
5668:
5564:
5270:
5167:
5103:
5007:
4999:
4857:
4130:
4027:
3934:
3915:
3847:
3825:
3786:
3419:
3300:
2865:
2660:
2416:
2098:
1733:
1308:
1222:
1199:
831:
31:
1818:, releasing carbon dioxide gas, which occurs when the sherbet is moistened by saliva.
1778:
noodles their characteristic flavour and chewy texture; a similar solution is used in
6279:
6238:
6192:
5802:
5709:
5688:
5637:
5581:
5463:
5223:
5183:
5151:
5119:
5028:
4823:
4776:
4221:
4193:
3964:
3926:
3907:
3814:
3774:
3734:
3615:
3588:
3531:
3506:
3444:
3411:
3268:
3212:
2992:
2984:
2923:
2813:
2761:
2670:
2652:
2509:
2420:
2292:
2262:
1787:
1753:
1667:
1655:
1612:
1214:
1173:
783:
698:
2249:, and coal. In the first step, sodium chloride is treated with sulfuric acid in the
1670:
is added to the melt mixture to make the glass insoluble. Bottle and window glass ("
6165:
5983:
5967:
5875:
5863:
5834:
5490:
5231:
5095:
5087:
5044:
4949:
4890:
4852:
4750:
4668:
4355:
4241:
4181:
3762:
3607:
3580:
3423:
3403:
3204:
3154:
3038:
2976:
2753:
2501:
2500:
The Solvay process recycles its ammonia. It consumes only brine and limestone, and
2250:
2075:
1962:
1861:
1857:
1830:
1811:
1772:
1741:
1605:
1226:
847:
744:
632:
576:
445:
3078:
2215:
plants in Spain. Plant and seaweed sources for soda ash, and also for the related
287:
6445:
6263:
6218:
6205:
6174:
5943:
5790:
5427:
5415:
5211:
5199:
4991:
4983:
4958:
4904:
4876:
4840:
4799:
4784:
4771:
3770:
3650:
3173:
2648:
2540:
2505:
2408:
2382:
2378:
2341:
2238:
2232:
1834:
1779:
1675:
1331:
2684:), means "coupled manufacturing alkali method": Hou's process is coupled to the
62:
6295:
6254:
6187:
5995:
5911:
5758:
5704:
5663:
5645:
5262:
5254:
5075:
5063:
5020:
5012:
4924:
4862:
4815:
4789:
4312:
4102:
2757:
2610:
2565:
2520:
2394:
2254:
2087:
2082:, trisodium hydrogendi carbonate dihydrate, are also known from ultra-alkaline
1974:
1795:
1601:
1424:
1339:
1251:
3042:
2980:
2729:
1161:
6496:
6394:
5846:
5036:
4794:
3711:
3611:
3463:
Grotzinger, J. and R. Milliken (eds.) 2012. Sedimentary
Geology of Mars. SEPM
3208:
3060:
3021:
Betzel, C.; Saenger, W.; Loewus, D. (1982). "Sodium
Carbonate Heptahydrate".
2988:
2843:
2765:
2685:
2475:
2404:
2242:
2039:
of sodium carbonate is −28.1 kJ/mol for a 10% w/w aqueous solution. The
2027:
Sodium carbonate is also used in the processing and tanning of animal hides.
1876:
1826:
1822:
1401:
1379:
892:
553:
266:
221:
3510:
1790:
bakers similarly use sodium carbonate as a substitute for lye-water to give
6381:
6157:
4941:
4933:
4831:
3415:
3291:
2996:
2689:
2188:
2106:
794:
647:
2950:
Dusek, Michal; Chapuis, Gervais; Meyer, Mathias; Petricek, Vaclav (2003).
2097:, interior material that has been brought to the surface. While there are
1134:
5510:
1815:
803:
488:
3407:
3158:
1120:
6462:
2536:
1691:
1456:. Soda ash is dissolved in water and crystallized to get washing soda.
1127:
789:
694:
680:
676:
658:
612:
464:
241:
201:
3696:
2809:
2543:
by-product carbon dioxide was pumped through a saturated solution of
2288:
2246:
2165:
2161:
2083:
1791:
1700:
1597:
1581:
1342:, as well as by carbonating sodium hydroxide which is made using the
1335:
767:
662:
3142:
1250:
Except where otherwise noted, data are given for materials in their
4719:
2673:, ammonium chloride is precipitated in a sodium chloride solution.
2588:
2581:
2572:
2516:
2212:
2193:
2169:
2021:
1880:
1803:
1093:
622:
258:
2870:(2nd ed.). New York: The MacMillan Company. pp. 208–209.
149:
2603:
2595:
2550:
2412:
2400:
2340:
The second stage is the reaction to produce sodium carbonate and
2316:
2312:
2208:
2178:
species) were harvested, dried, and burned. The ashes were then "
2051:
1799:
1355:
1323:
1128:
651:
643:
639:
627:
505:
274:
2063:) formed when seasonal lakes evaporate. Deposits of the mineral
2304:
2220:
2216:
2064:
1970:
1884:
1883:
water to maintain a desired pH and carbonate hardness (KH). In
1783:
1749:
1659:
1363:
1327:
618:
181:
2547:
and ammonia to produce sodium bicarbonate by these reactions:
2407:
developed a method to make sodium carbonate by first reacting
868:α = 90°, β = 101.35(8)°, γ = 90°
5589:
2617:
2557:
2544:
2308:
2241:
patented a process for producing sodium carbonate from salt,
2174:
2125:
2079:
2068:
1775:
1642:
1631:
1623:
1385:
sodium carbonate heptahydrate (not known in mineral form), Na
298:
161:
139:
87:
Soda ash, washing soda, soda crystals, sodium trioxocarbonate
3694:
3373:"Tatachemicals.com/north-america/product/images/fig_2_1.jpg"
1798:(and Central European cuisine more broadly), breads such as
865: = 6.050(5) Å (γ-form, anhydrous, 295 K)
356:
3140:
2864:
Comey, Arthur
Messinger; Hahn, Dorothy A. (February 1921).
2524:
2296:
2204:
2197:
1840:
1627:
1072:
495:
3093:"Material Safety Data Sheet – Sodium Carbonate, Anhydrous"
3474:"Ciner Weighs Sale of Stake in $ 5 Billion Soda Ash Unit"
3265:
Basic principles and calculations in chemical engineering
2949:
1897:
1737:
1571:{\displaystyle {\ce {Na2CO3 + 10H2O -> Na2CO3.10H2O}}}
1561:
1541:
1528:
1509:
1489:
1476:
1208:
1193:
3443:. Springer Science & Business Media. pp. 399–.
2746:
1973:
from cellulose. This reaction is exploited for removing
1969:), which is used for the "sulfite" method of separating
1961:
In a related reaction, sodium carbonate is used to make
3635:
2102:
1893:
1868:
1837:, and stabilizer. It is also used in the production of
1641:
It is used in the manufacture of sodium compounds like
2730:"Crystal Structure of Sodium Carbonate Monohydrate, Na
1794:
their characteristic texture and improve browning. In
1592:
Some common applications of sodium carbonate include:
406:
InChI=1/NaHCO3.2Na/c2-1(3)4;;/h(H2,2,3,4);;/q;2*+1/p-2
396:
InChI=1S/CH2O3.2Na/c2-1(3)4;;/h(H2,2,3,4);;/q;2*+1/p-2
1856:
Sodium carbonate is also used as a relatively strong
1464:
558:
851 °C (1,564 °F; 1,124 K) (Anhydrous)
259:
3505:(1848 ed.). London: Longman. pp. 1198–9.
3020:
2299:. This conversion entails two parts. First is the
1604:, which converts fats and grease to water-soluble
1570:
3436:
6494:
3296:"For Old-Fashioned Flavor, Bake the Baking Soda"
3198:
2867:A Dictionary of Chemical Solubilities: Inorganic
2046:
1924:, making it more economical to react finished Na
286:
3577:Kirk-Othmer Encyclopedia of Chemical Technology
3262:
2839:Solubilities of Inorganic and Organic Compounds
1903:
1838:
1757:
1109:
124:
116:
108:
3604:Ullmann's Encyclopedia of Industrial Chemistry
3526:Clow, Archibald and Clow, Nan L. (June 1952).
3263:Himmelblau, David M.; Riggs, James B. (2022).
3201:Ullmann's Encyclopedia of Industrial Chemistry
3199:Christian Thieme (2000). "Sodium Carbonates".
2535:This process was developed by Chinese chemist
1810:Sodium carbonate is used in the production of
1764:
4735:
3680:
2836:Seidell, Atherton; Linke, William F. (1919).
2381:was a major source of air pollution, and the
3585:10.1002/0471238961.1915040918012108.a01.pub3
2835:
2478:) left over from carbon dioxide generation:
1771:, a solution of alkaline salts used to give
1727:
811:C2/m, No. 12 (β-form, anhydrous, 628 K)
809:C2/m, No. 12 (γ-form, anhydrous, 170 K)
2883:
2881:
2879:
2877:
4742:
4728:
3687:
3673:
3430:
3385:
3232:
3230:
3228:
3194:
3192:
3190:
3188:
3186:
1888:immersion dyeing). It is also used in the
1845:to stabilize the pH of the final product.
817:/n, No. 14 (δ-form, anhydrous, 110 K)
371:
231:
209:
3522:
3520:
3258:
3256:
3254:
2863:
1550:
1498:
564:33.5 °C (92.3 °F; 306.6 K)
337:
329:
322:
3602:Thieme, C. (2000). "Sodium Carbonates".
3575:Eggeman, T. (2011). "Sodium Carbonate".
3286:
3284:
3147:Journal of the American Chemical Society
2913:
2911:
2909:
2887:
2874:
2415:, water, and carbon dioxide to generate
2050:
2043:of sodium carbonate monohydrate is 1.3.
1875:agents. It is also a common additive in
3641:International Chemical Safety Card 1135
3574:
3225:
3183:
3056:
3054:
3052:
2917:
2859:
2857:
2130:trisodium hydrogendicarbonate dihydrate
2071:and in the early manufacture of glass.
1821:Sodium carbonate also finds use in the
837:2/m (γ-form, β-form, δ-form, anhydrous)
367:
14:
6495:
3724:
3601:
3547:
3541:
3517:
3497:
3251:
3016:
3014:
3012:
2831:
2829:
2727:
2377:The hydrochloric acid produced by the
2055:Structure of monohydrate at 346 K
2030:
1635:
1354:Sodium carbonate is obtained as three
222:
4723:
4415:
3668:
3290:
3281:
3245:Cornell Center for Materials Research
3174:"On the hydrates of sodium carbonate"
3121:"Soda Ash Statistics and Information"
2945:
2943:
2941:
2939:
2906:
2801:CRC Handbook of Chemistry and Physics
2793:
2791:
2789:
2787:
2785:
2783:
2781:
2723:
2721:
2719:
2717:
1851:
560:100 °C (212 °F; 373 K)
399:Key: CDBYLPFSWZWCQE-UHFFFAOYSA-L
189:
169:
4749:
3171:
3141:T.W.Richards and A.H. Fiske (1914).
3076:
3049:
2854:
2797:
1649:
1616:
3123:. United States Geographical Survey
3085:
3009:
2846:: D. Van Nostrand Company. p.
2826:
2676:The Chinese name of Hou's process,
2155:
1977:from flue gases in power stations:
792:(γ-form, β-form, δ-form, anhydrous)
568:34 °C (93 °F; 307 K)
409:Key: CDBYLPFSWZWCQE-NUQVWONBAP
277:
24:
3568:
3437:Jeffrey S. Kargel (23 July 2004).
2936:
2778:
2714:
2226:
1900:and other mildly basic compounds.
1685:
1600:properties through the process of
1105:
34:(baking soda), a similar compound.
25:
6539:
3651:Use of sodium carbonate in dyeing
3636:American Natural Soda Ash Company
3629:
3548:Kiefer, David M. (January 2002).
2388:
1622:It is used in the manufacture of
479:g/mol (decahydrate)
3023:Acta Crystallographica Section B
2960:Acta Crystallographica Section B
2530:
2196:obtained from coastal plants or
2086:, that occur for example in the
2015:
1829:(E500) as an acidity regulator,
1440:Sodium carbonate decahydrate (Na
1258:
1042:
61:
52:
43:
3491:
3466:
3457:
3379:
3365:
3341:
3315:
3165:
3134:
3113:
3070:
2920:Handbook of Inorganic Chemicals
2702:Residual sodium carbonate index
2207:"), to 30 percent for the best
1896:as a float conditioner besides
1587:
1435:
1254:(at 25 °C , 100 kPa).
3656:Sodium carbonate manufacturing
3440:Mars - A Warmer, Wetter Planet
3100:conservationsupportsystems.com
2303:whereby the coal, a source of
2295:) was reduced by heating with
1515:
1400:sodium carbonate monohydrate (
1362:sodium carbonate decahydrate (
1006:Occupational safety and health
861: = 5.245(5) Å,
857: = 8.920(7) Å,
13:
1:
3349:"Home Tanning Hides and Furs"
2707:
2112:
2047:Occurrence as natural mineral
1867:For example, it is used as a
1654:Sodium carbonate serves as a
3102:. ConservationSupportSystems
2952:"Sodium carbonate revisited"
2922:. McGraw-Hill. p. 861.
2888:Anatolievich, Kiper Ruslan.
2798:Lide, David R., ed. (2009).
2237:In 1792, the French chemist
2172:) or the seaweed (typically
1904:Precursor to other compounds
1611:It is used for lowering the
825:Pbca, No. 61 (heptahydrate)
797:(monohydrate, heptahydrate)
671:g/100 g (155 °C)
517:g/cm (25 °C, anhydrous)
7:
2695:
1758:
1580:It is one of the few metal
1358:and as the anhydrous salt:
1349:
1150:or concentration (LD, LC):
881:Octahedral (Na, anhydrous)
841:2/m 2/m 2/m (heptahydrate)
689:g/100 g (20 °C)
10:
6544:
4417:
3067:. Retrieved on 2014-05-06.
2758:10.1524/zkri.1936.95.1.266
2539:in the 1930s. The earlier
2392:
2287:The salt cake and crushed
2230:
1689:
1584:that is soluble in water.
582:Anhydrous, g/100 mL:
29:
6156:
5475:
5247:
5056:
4971:
4917:
4808:
4764:
4757:
3726:
3704:
3695:Compounds containing the
3550:"It was all about alkali"
3043:10.1107/S0567740882009996
2981:10.1107/S0108768103009017
2918:Pradyot, Patnaik (2003).
2681:
2253:. This reaction produces
2120:
1892:to maintain a favourable
1765:
1728:Food additive and cooking
1248:
1185:
1146:
1023:
1003:
998:
885:
776:
566:decomposes (heptahydrate)
438:
418:
383:
267:(acidity regulators, ...)
92:
84:
74:
69:
60:
51:
42:
3612:10.1002/14356007.a24_299
3238:"Water Hardness Reading"
3209:10.1002/14356007.a24_299
1636:§ Glass manufacture
1454:water of crystallization
1382:to form the monohydrate.
1081:Precautionary statements
626:Slightly soluble in aq.
562:decomposes (monohydrate)
30:Not to be confused with
3646:FMC Wyoming Corporation
3554:Today's Chemist at Work
3203:. Weinheim: Wiley-VCH.
2366:This mixture is called
1890:froth flotation process
1786:, for similar reasons.
1168:4090 mg/kg (rat, oral)
732:Magnetic susceptibility
6513:Photographic chemicals
3658:by synthetic processes
2105:in previously aqueous
2056:
1839:
1748:). Alkalinity affects
1617:§ Water softening
1608:(specifically, soaps).
1572:
1239:Sodium sesquicarbonate
1116:
823:, No. 29 (monohydrate)
3329:. Janalta Interactive
3294:(24 September 2010).
2301:carbothermic reaction
2186:, in turn applied to
2095:bright spots on Ceres
2054:
1573:
1115:
876:Coordination geometry
772:3.4 cP (887 °C)
2150:Green River, Wyoming
2037:enthalpy of solution
1740:(which may refer to
1462:
1098:(fire diamond)
762:1.405 (decahydrate)
595:48.69 (34.8 °C)
592:34.07 (27.8 °C)
6508:Household chemicals
3530:. Ayer. pp. 65–90.
3528:Chemical Revolution
3408:10.1038/nature18290
3400:2016Natur.536...54D
3159:10.1021/ja02180a003
3077:Chambers, Michael.
3035:1982AcCrB..38.2802B
2973:2003AcCrB..59..337D
2806:Boca Raton, Florida
2403:industrial chemist
2031:Physical properties
1746:potassium hydroxide
1744:or, less commonly,
1563:
1543:
1530:
1511:
1491:
1478:
1344:chloralkali process
1243:Sodium percarbonate
1219:Potassium carbonate
760:1.420 (monohydrate)
598:48.1 (41.9 °C)
577:Solubility in water
538:g/cm (heptahydrate)
342: (decahydrate)
334: (monohydrate)
129: (decahydrate)
121: (monohydrate)
39:
6523:E-number additives
3323:"Sodium Carbonate"
3301:The New York Times
2890:"sodium carbonate"
2688:and offers better
2417:sodium bicarbonate
2099:carbonates on Mars
2057:
1852:Other applications
1736:) but weaker than
1734:sodium bicarbonate
1568:
1551:
1531:
1518:
1499:
1479:
1466:
1309:inorganic compound
1281:Infobox references
1223:Rubidium carbonate
1200:Sodium bicarbonate
1186:Related compounds
1117:
604:43.6 (100 °C)
601:45.62 (60 °C)
545:g/cm (decahydrate)
531:g/cm (monohydrate)
524:g/cm (856 °C)
37:
32:Sodium bicarbonate
6490:
6489:
6152:
6151:
4717:
4716:
4711:
4710:
3579:. pp. 1–11.
3450:978-1-85233-568-7
3274:978-0-13-732717-1
3061:Sigma-Aldrich Co.
3029:(11): 2802–2804.
2929:978-0-07-049439-8
2819:978-1-4200-9084-0
2804:(90th ed.).
2678:lianhe zhijian fa
2671:common-ion effect
2510:hydrogen chloride
2421:ammonium chloride
2293:calcium carbonate
2263:hydrogen chloride
1754:Maillard reaction
1668:calcium carbonate
1650:Glass manufacture
1613:hardness of water
1566:
1554:
1534:
1521:
1514:
1502:
1482:
1469:
1418:crystal carbonate
1416:O. Also known as
1378:O, which readily
1311:with the formula
1289:Chemical compound
1287:
1286:
1234:Related compounds
1215:Lithium carbonate
1174:Safety data sheet
1067:Hazard statements
975:Gibbs free energy
839:mm2 (monohydrate)
784:Crystal structure
758:1.485 (anhydrous)
699:dimethylformamide
589:16.4 (15 °C)
473:g/mol (anhydrous)
352:CompTox Dashboard
151:Interactive image
113: (anhydrous)
38:Sodium carbonate
27:Chemical compound
16:(Redirected from
6535:
6518:Sodium compounds
5560:
5546:
5536:
5248:Oxypnictogenides
5057:Oxychalcogenides
4762:
4761:
4751:Sodium compounds
4744:
4737:
4730:
4721:
4720:
3708:
3707:
3689:
3682:
3675:
3666:
3665:
3625:
3598:
3562:
3561:
3545:
3539:
3524:
3515:
3514:
3495:
3489:
3488:
3486:
3485:
3470:
3464:
3461:
3455:
3454:
3434:
3428:
3427:
3383:
3377:
3376:
3369:
3363:
3362:
3360:
3358:
3353:
3345:
3339:
3338:
3336:
3334:
3319:
3313:
3312:
3310:
3308:
3288:
3279:
3278:
3260:
3249:
3248:
3242:
3234:
3223:
3222:
3196:
3181:
3180:
3178:
3169:
3163:
3162:
3138:
3132:
3131:
3129:
3128:
3117:
3111:
3110:
3108:
3107:
3097:
3089:
3083:
3082:
3074:
3068:
3065:Sodium carbonate
3058:
3047:
3046:
3018:
3007:
3006:
3004:
3003:
2956:
2947:
2934:
2933:
2915:
2904:
2903:
2901:
2900:
2885:
2872:
2871:
2861:
2852:
2851:
2842:(2nd ed.).
2833:
2824:
2823:
2795:
2776:
2775:
2773:
2772:
2725:
2683:
2502:calcium chloride
2251:Mannheim process
2156:Barilla and kelp
2128:, also known as
2084:pegmatitic rocks
2076:Ol Doinyo Lengai
1963:sodium bisulfite
1862:sodium hydroxide
1844:
1831:anticaking agent
1770:
1768:
1767:
1761:
1742:sodium hydroxide
1721:
1715:
1714:
1711:
1577:
1575:
1574:
1569:
1567:
1564:
1562:
1559:
1552:
1542:
1539:
1532:
1529:
1526:
1519:
1512:
1510:
1507:
1500:
1490:
1487:
1480:
1477:
1474:
1467:
1322:and its various
1321:
1293:Sodium carbonate
1271:
1265:
1262:
1261:
1227:Cesium carbonate
1137:
1130:
1123:
1108:
1088:
1074:
1046:
993:
986:
967:
960:
936:
929:
908:
901:
886:Thermochemistry
848:Lattice constant
745:Refractive index
706:
688:
670:
544:
537:
530:
523:
516:
478:
472:
446:Chemical formula
376:
375:
360:
358:
341:
333:
326:
290:
279:
261:
243:
235:
224:
213:
193:
173:
153:
128:
120:
112:
79:Sodium carbonate
65:
56:
47:
40:
36:
21:
6543:
6542:
6538:
6537:
6536:
6534:
6533:
6532:
6493:
6492:
6491:
6486:
6482:
6478:
6474:
6470:
6466:
6457:
6453:
6449:
6440:
6436:
6427:
6423:
6415:
6411:
6402:
6398:
6389:
6385:
6376:
6372:
6368:
6360:
6356:
6352:
6344:
6340:
6336:
6327:
6319:
6315:
6307:
6303:
6299:
6291:
6287:
6283:
6275:
6271:
6267:
6258:
6250:
6246:
6242:
6234:
6230:
6226:
6222:
6213:
6209:
6200:
6196:
6182:
6178:
6169:
6148:
6144:
6140:
6132:
6128:
6124:
6115:
6111:
6107:
6099:
6095:
6087:
6083:
6075:
6071:
6067:
6063:
6055:
6051:
6043:
6039:
6031:
6027:
6023:
6015:
6011:
6003:
5999:
5991:
5987:
5979:
5975:
5971:
5963:
5959:
5951:
5947:
5939:
5935:
5931:
5923:
5919:
5915:
5907:
5903:
5895:
5891:
5883:
5879:
5871:
5867:
5859:
5850:
5842:
5838:
5830:
5826:
5822:
5814:
5810:
5806:
5798:
5794:
5786:
5782:
5778:
5770:
5766:
5762:
5754:
5750:
5742:
5734:
5726:
5713:
5700:
5692:
5684:
5680:
5672:
5659:
5641:
5633:
5629:
5625:
5617:
5613:
5609:
5601:
5597:
5593:
5585:
5576:
5568:
5559:
5555:
5551:
5545:
5541:
5535:
5531:
5526:
5518:
5514:
5506:
5502:
5494:
5486:
5471:
5467:
5459:
5455:
5447:
5443:
5435:
5431:
5423:
5419:
5411:
5407:
5403:
5399:
5391:
5387:
5383:
5375:
5371:
5367:
5359:
5355:
5347:
5343:
5339:
5330:
5326:
5318:
5314:
5306:
5302:
5294:
5290:
5282:
5278:
5274:
5266:
5258:
5243:
5239:
5235:
5227:
5219:
5215:
5207:
5203:
5195:
5191:
5187:
5179:
5175:
5171:
5163:
5159:
5155:
5147:
5143:
5139:
5131:
5127:
5123:
5115:
5111:
5107:
5099:
5091:
5083:
5079:
5071:
5067:
5052:
5048:
5040:
5032:
5024:
5016:
5003:
4995:
4987:
4967:
4962:
4953:
4945:
4937:
4928:
4913:
4908:
4894:
4880:
4866:
4848:
4844:
4835:
4827:
4819:
4804:
4780:
4753:
4748:
4718:
4713:
4712:
4676:
4672:
4661:
4657:
4641:
4637:
4633:
4625:
4621:
4617:
4609:
4605:
4601:
4593:
4589:
4585:
4577:
4573:
4569:
4561:
4557:
4553:
4545:
4541:
4537:
4529:
4525:
4521:
4516:
4513:
4505:
4501:
4497:
4486:
4482:
4478:
4470:
4466:
4462:
4454:
4450:
4446:
4438:
4434:
4430:
4359:
4340:
4336:
4328:
4324:
4316:
4308:
4304:
4296:
4264:
4260:
4256:
4245:
4237:
4232:
4229:
4225:
4197:
4189:
4185:
4177:
4146:
4138:
4134:
4106:
4098:
4094:
4090:
4083:
4078:
4075:
4071:
4063:
4055:
4051:
4047:
4042:
4039:
4031:
4023:
4015:
4011:
4007:
4002:
3999:
3980:
3976:
3971:
3968:
3960:
3955:
3952:
3948:
3930:
3919:
3914:
3911:
3903:
3899:
3895:
3887:
3883:
3878:
3875:
3867:
3863:
3859:
3854:
3851:
3846:
3843:
3839:
3818:
3813:
3810:
3806:
3801:
3798:
3794:
3790:
3782:
3778:
3773:
3766:
3758:
3750:
3745:
3742:
3738:
3719:
3715:
3700:
3693:
3662:
3632:
3622:
3595:
3571:
3569:Further reading
3566:
3565:
3546:
3542:
3525:
3518:
3503:Lexicon Medicum
3496:
3492:
3483:
3481:
3472:
3471:
3467:
3462:
3458:
3451:
3435:
3431:
3394:(7614): 54–57.
3384:
3380:
3371:
3370:
3366:
3356:
3354:
3351:
3347:
3346:
3342:
3332:
3330:
3321:
3320:
3316:
3306:
3304:
3289:
3282:
3275:
3261:
3252:
3240:
3236:
3235:
3226:
3219:
3197:
3184:
3176:
3170:
3166:
3139:
3135:
3126:
3124:
3119:
3118:
3114:
3105:
3103:
3095:
3091:
3090:
3086:
3075:
3071:
3059:
3050:
3019:
3010:
3001:
2999:
2954:
2948:
2937:
2930:
2916:
2907:
2898:
2896:
2886:
2875:
2862:
2855:
2834:
2827:
2820:
2796:
2779:
2770:
2768:
2741:
2737:
2733:
2726:
2715:
2710:
2698:
2666:
2664:
2656:
2645:
2641:
2635:
2633:
2629:
2621:
2614:
2607:
2601:
2599:
2592:
2585:
2578:
2576:
2569:
2561:
2554:
2545:sodium chloride
2541:steam reforming
2533:
2506:calcium sulfide
2498:
2496:
2492:
2488:
2484:
2472:
2471:
2467:
2463:
2459:
2455:
2447:
2445:
2441:
2437:
2433:
2429:
2409:sodium chloride
2397:
2391:
2383:calcium sulfide
2379:Leblanc process
2364:
2362:
2358:
2354:
2350:
2342:calcium sulfide
2338:
2337:
2333:
2329:
2325:
2285:
2283:
2279:
2275:
2271:
2239:Nicolas Leblanc
2235:
2233:Leblanc process
2229:
2227:Leblanc process
2158:
2147:
2143:
2139:
2135:
2123:
2115:
2049:
2033:
2018:
2004:
2003:
1999:
1995:
1991:
1987:
1983:
1968:
1959:
1958:
1954:
1950:
1946:
1942:
1935:
1931:
1927:
1923:
1919:
1915:
1906:
1873:film developing
1854:
1780:Chinese cuisine
1762:
1730:
1722:
1719:
1712:
1709:
1708:
1706:
1694:
1688:
1686:Water softening
1681:
1676:silicon dioxide
1672:soda–lime glass
1665:
1652:
1590:
1560:
1555:
1540:
1535:
1527:
1522:
1508:
1503:
1488:
1483:
1475:
1470:
1465:
1463:
1460:
1459:
1451:
1447:
1443:
1438:
1415:
1411:
1407:
1396:
1392:
1388:
1377:
1373:
1369:
1352:
1332:sodium chloride
1320:
1316:
1312:
1295:(also known as
1290:
1283:
1278:
1277:
1276: ?)
1267:
1263:
1259:
1255:
1241:
1235:
1225:
1221:
1217:
1211:
1196:
1165:
1159:
1142:
1141:
1140:
1139:
1132:
1125:
1118:
1114:
1106:
1083:
1069:
1055:
1039:
1016:
991:
987:
981:
977:
965:
961:
958:
952:
948:
945:
944:Std enthalpy of
934:
930:
927:
920:
917:
906:
902:
895:
878:
869:
866:
850:
840:
838:
834:
824:
822:
818:
816:
812:
810:
806:
793:
786:
761:
759:
755:
753:
738:−4.1·10 cm/mol
735:
721:
704:
686:
668:
636:
630:
625:
617:Soluble in aq.
607:
579:
569:
567:
565:
563:
561:
559:
548:
542:
535:
528:
521:
514:
476:
474:
470:
458:
454:
448:
434:
431:
426:
425:
414:
411:
410:
407:
401:
400:
397:
391:
390:
379:
361:
354:
345:
309:
293:
280:
253:
216:
196:
176:
156:
143:
132:
102:
88:
80:
35:
28:
23:
22:
15:
12:
11:
5:
6541:
6531:
6530:
6525:
6520:
6515:
6510:
6505:
6488:
6487:
6485:
6484:
6480:
6476:
6472:
6468:
6464:
6460:
6455:
6451:
6447:
6443:
6438:
6434:
6430:
6425:
6421:
6417:
6413:
6409:
6405:
6400:
6396:
6392:
6387:
6383:
6379:
6374:
6370:
6366:
6362:
6358:
6354:
6350:
6346:
6342:
6338:
6334:
6330:
6325:
6321:
6317:
6313:
6309:
6305:
6301:
6297:
6293:
6289:
6285:
6281:
6277:
6273:
6269:
6265:
6261:
6256:
6252:
6248:
6244:
6240:
6236:
6232:
6228:
6224:
6220:
6216:
6211:
6207:
6203:
6198:
6194:
6190:
6185:
6180:
6176:
6172:
6167:
6162:
6160:
6154:
6153:
6150:
6149:
6147:
6146:
6142:
6138:
6134:
6130:
6126:
6122:
6118:
6113:
6109:
6105:
6101:
6097:
6093:
6089:
6085:
6081:
6077:
6073:
6069:
6065:
6061:
6057:
6053:
6049:
6045:
6041:
6037:
6033:
6029:
6025:
6021:
6017:
6013:
6009:
6005:
6001:
5997:
5993:
5989:
5985:
5981:
5977:
5973:
5969:
5965:
5961:
5957:
5953:
5949:
5945:
5941:
5937:
5933:
5929:
5925:
5921:
5917:
5913:
5909:
5905:
5901:
5897:
5893:
5889:
5885:
5881:
5877:
5873:
5869:
5865:
5861:
5857:
5853:
5848:
5844:
5840:
5836:
5832:
5828:
5824:
5820:
5816:
5812:
5808:
5804:
5800:
5796:
5792:
5788:
5784:
5780:
5776:
5772:
5768:
5764:
5760:
5756:
5752:
5748:
5744:
5740:
5736:
5732:
5728:
5724:
5720:
5715:
5711:
5707:
5702:
5698:
5694:
5690:
5686:
5682:
5678:
5674:
5670:
5666:
5661:
5657:
5653:
5648:
5643:
5639:
5635:
5631:
5627:
5623:
5619:
5615:
5611:
5607:
5603:
5599:
5595:
5591:
5587:
5583:
5579:
5574:
5570:
5566:
5562:
5557:
5553:
5548:
5543:
5538:
5533:
5528:
5524:
5520:
5516:
5512:
5508:
5504:
5500:
5496:
5492:
5488:
5484:
5479:
5477:
5473:
5472:
5470:
5469:
5465:
5461:
5457:
5453:
5449:
5445:
5441:
5437:
5433:
5429:
5425:
5421:
5417:
5413:
5409:
5405:
5401:
5397:
5393:
5389:
5385:
5381:
5377:
5373:
5369:
5365:
5361:
5357:
5353:
5349:
5345:
5341:
5337:
5333:
5328:
5324:
5320:
5316:
5312:
5308:
5304:
5300:
5296:
5292:
5288:
5284:
5280:
5276:
5272:
5268:
5264:
5260:
5256:
5251:
5249:
5245:
5244:
5242:
5241:
5237:
5233:
5229:
5225:
5221:
5217:
5213:
5209:
5205:
5201:
5197:
5193:
5189:
5185:
5181:
5177:
5173:
5169:
5165:
5161:
5157:
5153:
5149:
5145:
5141:
5137:
5133:
5129:
5125:
5121:
5117:
5113:
5109:
5105:
5101:
5097:
5093:
5089:
5085:
5081:
5077:
5073:
5069:
5065:
5060:
5058:
5054:
5053:
5051:
5050:
5046:
5042:
5038:
5034:
5030:
5026:
5022:
5018:
5014:
5010:
5005:
5001:
4997:
4993:
4989:
4985:
4981:
4975:
4973:
4969:
4968:
4966:
4965:
4960:
4956:
4951:
4947:
4943:
4939:
4935:
4931:
4926:
4921:
4919:
4915:
4914:
4912:
4911:
4906:
4902:
4897:
4892:
4888:
4883:
4878:
4874:
4869:
4864:
4860:
4855:
4850:
4846:
4842:
4838:
4833:
4829:
4825:
4821:
4817:
4812:
4810:
4806:
4805:
4803:
4802:
4797:
4792:
4787:
4782:
4778:
4774:
4768:
4766:
4759:
4755:
4754:
4747:
4746:
4739:
4732:
4724:
4715:
4714:
4709:
4708:
4705:
4702:
4699:
4696:
4693:
4690:
4687:
4684:
4681:
4678:
4674:
4670:
4666:
4663:
4659:
4655:
4651:
4648:
4644:
4643:
4639:
4635:
4631:
4627:
4623:
4619:
4615:
4611:
4607:
4603:
4599:
4595:
4591:
4587:
4583:
4579:
4575:
4571:
4567:
4563:
4559:
4555:
4551:
4547:
4543:
4539:
4535:
4531:
4527:
4523:
4519:
4511:
4507:
4503:
4499:
4495:
4491:
4488:
4484:
4480:
4476:
4472:
4468:
4464:
4460:
4456:
4452:
4448:
4444:
4440:
4436:
4432:
4428:
4424:
4420:
4419:
4416:
4413:
4412:
4409:
4406:
4403:
4400:
4397:
4394:
4391:
4388:
4385:
4382:
4379:
4376:
4373:
4370:
4367:
4364:
4361:
4357:
4353:
4349:
4348:
4345:
4342:
4338:
4334:
4330:
4326:
4322:
4318:
4314:
4310:
4306:
4302:
4298:
4294:
4290:
4287:
4284:
4281:
4278:
4275:
4272:
4269:
4266:
4262:
4258:
4254:
4250:
4247:
4243:
4239:
4235:
4227:
4223:
4218:
4217:
4214:
4211:
4208:
4205:
4202:
4199:
4195:
4191:
4187:
4183:
4179:
4175:
4171:
4168:
4165:
4162:
4159:
4156:
4153:
4150:
4148:
4144:
4140:
4136:
4132:
4127:
4126:
4123:
4120:
4117:
4114:
4111:
4108:
4104:
4100:
4096:
4092:
4088:
4081:
4073:
4069:
4065:
4061:
4057:
4053:
4049:
4045:
4037:
4033:
4029:
4025:
4021:
4017:
4013:
4009:
4005:
3997:
3993:
3990:
3987:
3984:
3982:
3978:
3974:
3966:
3962:
3958:
3950:
3946:
3941:
3940:
3937:
3932:
3928:
3924:
3921:
3917:
3909:
3905:
3901:
3897:
3893:
3889:
3885:
3881:
3873:
3869:
3865:
3861:
3857:
3849:
3841:
3837:
3832:
3831:
3828:
3823:
3820:
3816:
3808:
3804:
3796:
3792:
3788:
3784:
3780:
3776:
3768:
3764:
3760:
3756:
3752:
3748:
3740:
3736:
3731:
3730:
3727:
3725:
3723:
3721:
3717:
3713:
3706:
3705:
3702:
3701:
3692:
3691:
3684:
3677:
3669:
3660:
3659:
3653:
3648:
3643:
3638:
3631:
3630:External links
3628:
3627:
3626:
3621:978-3527306732
3620:
3599:
3594:978-0471238966
3593:
3570:
3567:
3564:
3563:
3540:
3516:
3499:Hooper, Robert
3490:
3465:
3456:
3449:
3429:
3378:
3364:
3340:
3327:corrosionpedia
3314:
3280:
3273:
3250:
3224:
3218:978-3527306732
3217:
3182:
3164:
3153:(3): 485–490.
3133:
3112:
3084:
3069:
3048:
3008:
2967:(3): 337–352.
2935:
2928:
2905:
2873:
2853:
2825:
2818:
2777:
2752:(1): 266–273.
2739:
2735:
2731:
2712:
2711:
2709:
2706:
2705:
2704:
2697:
2694:
2662:
2654:
2643:
2639:
2636:
2631:
2627:
2619:
2612:
2605:
2602:
2597:
2590:
2583:
2579:
2574:
2567:
2559:
2552:
2549:
2532:
2529:
2521:alkali noodles
2494:
2490:
2486:
2485:Cl + CaO → 2NH
2482:
2480:
2469:
2465:
2461:
2457:
2453:
2451:
2443:
2439:
2435:
2431:
2427:
2425:
2395:Solvay process
2393:Main article:
2390:
2389:Solvay process
2387:
2360:
2356:
2352:
2348:
2346:
2335:
2331:
2327:
2323:
2321:
2281:
2277:
2273:
2269:
2267:
2255:sodium sulfate
2231:Main article:
2228:
2225:
2211:produced from
2157:
2154:
2145:
2141:
2137:
2133:
2122:
2119:
2114:
2111:
2088:Kola Peninsula
2048:
2045:
2032:
2029:
2017:
2014:
2001:
1997:
1993:
1989:
1985:
1981:
1979:
1975:sulfur dioxide
1966:
1956:
1952:
1948:
1944:
1940:
1938:
1933:
1929:
1925:
1921:
1917:
1913:
1911:
1905:
1902:
1877:swimming pools
1853:
1850:
1796:German cuisine
1729:
1726:
1717:
1705:
1687:
1684:
1679:
1663:
1651:
1648:
1647:
1646:
1639:
1620:
1609:
1602:saponification
1589:
1586:
1558:
1549:
1546:
1538:
1525:
1517:
1506:
1497:
1494:
1486:
1473:
1449:
1445:
1441:
1437:
1434:
1429:
1428:
1425:Solvay process
1421:
1413:
1409:
1405:
1398:
1394:
1390:
1386:
1383:
1375:
1371:
1367:
1351:
1348:
1340:Solvay process
1318:
1314:
1288:
1285:
1284:
1279:
1257:
1256:
1252:standard state
1249:
1246:
1245:
1236:
1233:
1230:
1229:
1212:
1206:
1203:
1202:
1197:
1191:
1188:
1187:
1183:
1182:
1177:
1170:
1169:
1166:
1157:
1155:
1152:
1151:
1144:
1143:
1133:
1126:
1119:
1104:
1103:
1102:
1101:
1099:
1090:
1089:
1087:P305+P351+P338
1084:
1079:
1076:
1075:
1070:
1065:
1062:
1061:
1056:
1051:
1048:
1047:
1040:
1035:
1032:
1031:
1021:
1020:
1017:
1014:
1011:
1010:
1001:
1000:
996:
995:
988:
979:
973:
970:
969:
962:
956:
950:
942:
939:
938:
931:
925:
914:
911:
910:
903:
891:
888:
887:
883:
882:
879:
874:
871:
870:
867:
853:
851:
846:
843:
842:
835:
830:
827:
826:
820:
814:
807:
802:
799:
798:
787:
782:
779:
778:
774:
773:
770:
764:
763:
756:
751:
743:
740:
739:
736:
730:
727:
726:
723:
719:
709:
708:
701:
691:
690:
683:
673:
672:
665:
655:
654:
634:
615:
609:
608:
606:
605:
602:
599:
596:
593:
590:
587:
583:
580:
575:
572:
571:
570:(decahydrate)
556:
550:
549:
547:
546:
539:
532:
525:
518:
510:
508:
502:
501:
498:
492:
491:
485:
481:
480:
467:
461:
460:
456:
452:
449:
444:
441:
440:
436:
435:
433:
432:
429:
421:
420:
419:
416:
415:
413:
412:
408:
405:
404:
402:
398:
395:
394:
386:
385:
384:
381:
380:
378:
377:
364:
362:
350:
347:
346:
344:
343:
335:
327:
319:
317:
311:
310:
308:
307:
303:
301:
295:
294:
292:
291:
283:
281:
273:
270:
269:
263:
255:
254:
252:
251:
247:
245:
237:
236:
226:
218:
217:
215:
214:
206:
204:
198:
197:
195:
194:
186:
184:
178:
177:
175:
174:
166:
164:
158:
157:
155:
154:
146:
144:
137:
134:
133:
131:
130:
122:
114:
105:
103:
98:
95:
94:
90:
89:
86:
82:
81:
78:
72:
71:
67:
66:
58:
57:
49:
48:
26:
9:
6:
4:
3:
2:
6540:
6529:
6526:
6524:
6521:
6519:
6516:
6514:
6511:
6509:
6506:
6504:
6501:
6500:
6498:
6483:
6461:
6459:
6444:
6442:
6431:
6429:
6418:
6416:
6406:
6404:
6393:
6391:
6380:
6378:
6363:
6361:
6347:
6345:
6331:
6329:
6322:
6320:
6310:
6308:
6294:
6292:
6278:
6276:
6262:
6260:
6253:
6251:
6237:
6235:
6217:
6215:
6204:
6202:
6191:
6189:
6186:
6184:
6173:
6171:
6164:
6163:
6161:
6159:
6155:
6145:
6135:
6133:
6119:
6117:
6102:
6100:
6090:
6088:
6078:
6076:
6058:
6056:
6046:
6044:
6034:
6032:
6018:
6016:
6006:
6004:
5994:
5992:
5982:
5980:
5966:
5964:
5954:
5952:
5942:
5940:
5926:
5924:
5910:
5908:
5898:
5896:
5886:
5884:
5874:
5872:
5862:
5860:
5854:
5852:
5845:
5843:
5833:
5831:
5817:
5815:
5801:
5799:
5789:
5787:
5773:
5771:
5757:
5755:
5745:
5743:
5737:
5735:
5729:
5727:
5721:
5719:
5716:
5714:
5708:
5706:
5703:
5701:
5695:
5693:
5687:
5685:
5675:
5673:
5667:
5665:
5662:
5660:
5654:
5652:
5649:
5647:
5644:
5642:
5636:
5634:
5620:
5618:
5604:
5602:
5588:
5586:
5580:
5578:
5571:
5569:
5563:
5561:
5549:
5547:
5539:
5537:
5529:
5527:
5521:
5519:
5509:
5507:
5497:
5495:
5489:
5487:
5481:
5480:
5478:
5474:
5468:
5462:
5460:
5450:
5448:
5438:
5436:
5426:
5424:
5414:
5412:
5394:
5392:
5378:
5376:
5362:
5360:
5350:
5348:
5334:
5332:
5321:
5319:
5309:
5307:
5297:
5295:
5285:
5283:
5269:
5267:
5261:
5259:
5253:
5252:
5250:
5246:
5240:
5230:
5228:
5222:
5220:
5210:
5208:
5198:
5196:
5182:
5180:
5166:
5164:
5150:
5148:
5134:
5132:
5118:
5116:
5102:
5100:
5094:
5092:
5086:
5084:
5074:
5072:
5062:
5061:
5059:
5055:
5049:
5043:
5041:
5035:
5033:
5027:
5025:
5019:
5017:
5011:
5009:
5006:
5004:
4998:
4996:
4990:
4988:
4982:
4980:
4977:
4976:
4974:
4970:
4964:
4957:
4955:
4948:
4946:
4940:
4938:
4932:
4930:
4923:
4922:
4920:
4918:Pnictogenides
4916:
4910:
4903:
4901:
4898:
4896:
4889:
4887:
4884:
4882:
4875:
4873:
4870:
4868:
4861:
4859:
4856:
4854:
4851:
4849:
4839:
4837:
4830:
4828:
4822:
4820:
4814:
4813:
4811:
4809:Chalcogenides
4807:
4801:
4798:
4796:
4793:
4791:
4788:
4786:
4783:
4781:
4775:
4773:
4770:
4769:
4767:
4763:
4760:
4756:
4752:
4745:
4740:
4738:
4733:
4731:
4726:
4725:
4722:
4706:
4703:
4700:
4697:
4694:
4691:
4688:
4685:
4682:
4679:
4677:
4667:
4664:
4662:
4652:
4649:
4646:
4645:
4642:
4628:
4626:
4612:
4610:
4596:
4594:
4580:
4578:
4564:
4562:
4548:
4546:
4532:
4530:
4514:
4508:
4506:
4492:
4489:
4487:
4473:
4471:
4457:
4455:
4441:
4439:
4425:
4422:
4421:
4414:
4410:
4407:
4404:
4401:
4398:
4395:
4392:
4389:
4386:
4383:
4380:
4377:
4374:
4371:
4368:
4365:
4362:
4360:
4354:
4351:
4350:
4346:
4343:
4341:
4331:
4329:
4319:
4317:
4311:
4309:
4299:
4297:
4291:
4288:
4285:
4282:
4279:
4276:
4273:
4270:
4267:
4265:
4251:
4248:
4246:
4240:
4238:
4230:
4220:
4219:
4215:
4212:
4209:
4206:
4203:
4200:
4198:
4192:
4190:
4180:
4178:
4172:
4169:
4166:
4163:
4160:
4157:
4154:
4151:
4149:
4147:
4141:
4139:
4129:
4128:
4124:
4121:
4118:
4115:
4112:
4109:
4107:
4101:
4099:
4084:
4076:
4066:
4064:
4058:
4056:
4040:
4034:
4032:
4026:
4024:
4018:
4016:
4000:
3994:
3991:
3988:
3985:
3983:
3981:
3969:
3963:
3961:
3953:
3943:
3942:
3938:
3936:
3933:
3931:
3925:
3922:
3920:
3912:
3906:
3904:
3890:
3888:
3876:
3870:
3868:
3852:
3844:
3834:
3833:
3829:
3827:
3824:
3821:
3819:
3811:
3799:
3785:
3783:
3772:
3769:
3767:
3761:
3759:
3753:
3751:
3743:
3733:
3732:
3728:
3722:
3720:
3710:
3709:
3703:
3698:
3690:
3685:
3683:
3678:
3676:
3671:
3670:
3667:
3663:
3657:
3654:
3652:
3649:
3647:
3644:
3642:
3639:
3637:
3634:
3633:
3623:
3617:
3613:
3609:
3605:
3600:
3596:
3590:
3586:
3582:
3578:
3573:
3572:
3559:
3555:
3551:
3544:
3537:
3536:0-8369-1909-2
3533:
3529:
3523:
3521:
3512:
3508:
3504:
3500:
3494:
3479:
3478:Bloomberg.com
3475:
3469:
3460:
3452:
3446:
3442:
3441:
3433:
3425:
3421:
3417:
3413:
3409:
3405:
3401:
3397:
3393:
3389:
3382:
3374:
3368:
3350:
3344:
3328:
3324:
3318:
3303:
3302:
3297:
3293:
3292:McGee, Harold
3287:
3285:
3276:
3270:
3266:
3259:
3257:
3255:
3246:
3239:
3233:
3231:
3229:
3220:
3214:
3210:
3206:
3202:
3195:
3193:
3191:
3189:
3187:
3175:
3168:
3160:
3156:
3152:
3148:
3144:
3137:
3122:
3116:
3101:
3094:
3088:
3080:
3073:
3066:
3062:
3057:
3055:
3053:
3044:
3040:
3036:
3032:
3028:
3024:
3017:
3015:
3013:
2998:
2994:
2990:
2986:
2982:
2978:
2974:
2970:
2966:
2962:
2961:
2953:
2946:
2944:
2942:
2940:
2931:
2925:
2921:
2914:
2912:
2910:
2895:
2891:
2884:
2882:
2880:
2878:
2869:
2868:
2860:
2858:
2849:
2845:
2841:
2840:
2832:
2830:
2821:
2815:
2811:
2807:
2803:
2802:
2794:
2792:
2790:
2788:
2786:
2784:
2782:
2767:
2763:
2759:
2755:
2751:
2747:
2743:
2724:
2722:
2720:
2718:
2713:
2703:
2700:
2699:
2693:
2691:
2687:
2686:Haber process
2679:
2674:
2672:
2665:
2658:
2650:
2646:
2634:
2623:
2615:
2608:
2600:
2593:
2586:
2577:
2570:
2563:
2555:
2548:
2546:
2542:
2538:
2531:Hou's process
2528:
2526:
2522:
2518:
2513:
2511:
2507:
2503:
2479:
2477:
2476:calcium oxide
2450:
2424:
2422:
2418:
2414:
2410:
2406:
2405:Ernest Solvay
2402:
2399:In 1861, the
2396:
2386:
2384:
2380:
2375:
2373:
2369:
2345:
2343:
2320:
2318:
2314:
2310:
2306:
2302:
2298:
2294:
2290:
2266:
2264:
2260:
2256:
2252:
2248:
2244:
2243:sulfuric acid
2240:
2234:
2224:
2222:
2218:
2214:
2210:
2206:
2201:
2199:
2195:
2191:
2190:
2185:
2181:
2177:
2176:
2171:
2167:
2163:
2153:
2151:
2131:
2127:
2118:
2110:
2108:
2104:
2100:
2096:
2091:
2089:
2085:
2081:
2077:
2072:
2070:
2066:
2062:
2053:
2044:
2042:
2041:Mohs hardness
2038:
2035:The integral
2028:
2025:
2023:
2016:Miscellaneous
2013:
2010:
2007:
1978:
1976:
1972:
1964:
1937:
1909:
1901:
1899:
1895:
1891:
1886:
1882:
1878:
1874:
1870:
1865:
1863:
1859:
1849:
1846:
1843:
1842:
1836:
1835:raising agent
1832:
1828:
1827:food additive
1824:
1823:food industry
1819:
1817:
1813:
1808:
1805:
1801:
1797:
1793:
1789:
1785:
1781:
1777:
1774:
1760:
1755:
1751:
1747:
1743:
1739:
1735:
1725:
1704:
1702:
1697:
1693:
1683:
1677:
1673:
1669:
1661:
1657:
1644:
1640:
1637:
1633:
1629:
1625:
1621:
1618:
1614:
1610:
1607:
1603:
1599:
1595:
1594:
1593:
1585:
1583:
1578:
1556:
1547:
1544:
1536:
1523:
1504:
1495:
1492:
1484:
1471:
1457:
1455:
1433:
1426:
1422:
1419:
1403:
1402:thermonatrite
1399:
1384:
1381:
1365:
1361:
1360:
1359:
1357:
1347:
1345:
1341:
1337:
1333:
1329:
1325:
1310:
1306:
1305:soda crystals
1302:
1298:
1294:
1282:
1275:
1270:
1253:
1247:
1244:
1240:
1237:
1232:
1231:
1228:
1224:
1220:
1216:
1213:
1210:
1205:
1204:
1201:
1198:
1195:
1190:
1189:
1184:
1181:
1178:
1175:
1172:
1171:
1167:
1163:
1154:
1153:
1149:
1145:
1138:
1131:
1124:
1100:
1097:
1096:
1092:
1091:
1085:
1082:
1078:
1077:
1071:
1068:
1064:
1063:
1060:
1057:
1054:
1050:
1049:
1045:
1041:
1038:
1034:
1033:
1029:
1027:
1022:
1018:
1013:
1012:
1008:
1007:
1002:
997:
989:
984:
976:
972:
971:
963:
955:
947:
941:
940:
932:
924:
919:
913:
912:
904:
899:
894:
893:Heat capacity
890:
889:
884:
880:
877:
873:
872:
864:
860:
856:
852:
849:
845:
844:
836:
833:
829:
828:
808:
805:
801:
800:
796:
791:
788:
785:
781:
780:
775:
771:
769:
766:
765:
757:
750:
746:
742:
741:
737:
733:
729:
728:
724:
718:
714:
711:
710:
702:
700:
696:
693:
692:
684:
682:
678:
675:
674:
666:
664:
660:
657:
656:
653:
649:
645:
641:
637:
631:Insoluble in
629:
624:
620:
616:
614:
611:
610:
603:
600:
597:
594:
591:
588:
586:7 (0 °C)
585:
584:
581:
578:
574:
573:
557:
555:
554:Melting point
552:
551:
540:
533:
526:
519:
512:
511:
509:
507:
504:
503:
499:
497:
494:
493:
490:
487:White solid,
486:
483:
482:
468:
466:
463:
462:
450:
447:
443:
442:
437:
428:
427:
424:
417:
403:
393:
392:
389:
382:
374:
370:
369:DTXSID1029621
366:
365:
363:
353:
349:
348:
340:
336:
332:
328:
325:
321:
320:
318:
316:
313:
312:
305:
304:
302:
300:
297:
296:
289:
285:
284:
282:
276:
272:
271:
268:
264:
262:
257:
256:
249:
248:
246:
244:
239:
238:
234:
230:
227:
225:
223:ECHA InfoCard
220:
219:
212:
208:
207:
205:
203:
200:
199:
192:
188:
187:
185:
183:
180:
179:
172:
168:
167:
165:
163:
160:
159:
152:
148:
147:
145:
141:
136:
135:
127:
123:
119:
115:
111:
107:
106:
104:
101:
97:
96:
91:
83:
77:
73:
68:
64:
59:
55:
50:
46:
41:
33:
19:
6528:Types of ash
5746:
3835:
3661:
3603:
3576:
3557:
3553:
3543:
3527:
3502:
3493:
3482:. Retrieved
3480:. 2021-08-09
3477:
3468:
3459:
3439:
3432:
3391:
3387:
3381:
3367:
3355:. Retrieved
3343:
3331:. Retrieved
3326:
3317:
3305:. Retrieved
3299:
3264:
3244:
3200:
3167:
3150:
3146:
3136:
3125:. Retrieved
3115:
3104:. Retrieved
3099:
3087:
3072:
3026:
3022:
3000:. Retrieved
2964:
2958:
2919:
2897:. Retrieved
2894:chemister.ru
2893:
2866:
2838:
2799:
2769:. Retrieved
2749:
2745:
2690:atom economy
2677:
2675:
2667:
2534:
2514:
2499:
2473:
2448:
2398:
2376:
2367:
2365:
2339:
2286:
2258:
2236:
2202:
2189:Salsola soda
2187:
2183:
2173:
2159:
2124:
2116:
2107:Martian soil
2092:
2073:
2060:
2058:
2034:
2026:
2019:
2011:
2008:
2005:
1960:
1907:
1866:
1855:
1847:
1820:
1809:
1731:
1723:
1698:
1695:
1653:
1591:
1588:Applications
1579:
1458:
1439:
1436:Washing soda
1430:
1417:
1353:
1304:
1300:
1297:washing soda
1296:
1292:
1291:
1147:
1094:
1058:
1025:
1015:Main hazards
1004:
982:
953:
922:
897:
862:
858:
854:
795:Orthorhombic
748:
716:
648:benzonitrile
299:RTECS number
191:ChEMBL186314
93:Identifiers
85:Other names
3771:(RO)(R'O)CO
2372:lixiviating
2090:in Russia.
1910:bicarbonate
1816:citric acid
1380:effloresces
1162:median dose
1148:Lethal dose
1053:Signal word
1009:(OHS/OSH):
832:Point group
804:Space group
646:, alcohol,
489:hygroscopic
484:Appearance
439:Properties
229:100.007.127
171:CHEBI:29377
6503:Carbonates
6497:Categories
4972:Oxyhalides
3560:(1): 45–6.
3484:2023-12-04
3333:9 November
3172:A. Pabst.
3127:2024-03-03
3106:2014-07-25
3002:2014-07-25
2899:2014-07-25
2771:2014-07-25
2708:References
2537:Hou Debang
2166:glassworts
2113:Production
2061:evaporites
1955:O → 2NaHCO
1792:moon cakes
1692:Hard water
1690:See also:
1582:carbonates
1037:Pictograms
790:Monoclinic
777:Structure
695:Solubility
681:ethanediol
677:Solubility
659:Solubility
613:Solubility
465:Molar mass
339:LS505BG22I
331:2A1Q1Q3557
324:45P3261C7T
202:ChemSpider
138:3D model (
100:CAS Number
76:IUPAC name
4758:Inorganic
3697:carbonate
2989:0108-7681
2810:CRC Press
2766:2196-7105
2438:O → NaHCO
2426:NaCl + NH
2368:black ash
2330:+ 2C → Na
2289:limestone
2268:2NaCl + H
2259:salt cake
2247:limestone
2180:lixivated
2170:saltworts
2162:halophyte
2160:Several "
1996:O → NaHCO
1804:lye rolls
1788:Cantonese
1707:Ca + CO
1701:carbonate
1598:detergent
1545:⋅
1516:⟶
1336:limestone
1307:) is the
1028:labelling
1019:Irritant
946:formation
916:Std molar
768:Viscosity
663:glycerine
650:, liquid
500:Odorless
306:VZ4050000
250:207-838-8
242:EC Number
126:6132-02-1
118:5968-11-6
3511:27671024
3501:(1802).
3416:27362221
3357:16 April
3307:25 April
2997:12761404
2844:New York
2696:See also
2517:pretzels
2351:S + CaCO
2213:saltwort
2022:alginate
1881:aquarium
1800:pretzels
1782:to make
1773:Japanese
1356:hydrates
1350:Hydrates
1324:hydrates
1301:soda ash
1095:NFPA 704
999:Hazards
937:J/mol·K
909:J/mol·K
734:(χ)
644:acetates
642:, alkyl
623:glycerol
475:286.1416
469:105.9888
265:E500(i)
260:E number
110:497-19-8
18:Soda ash
6158:Organic
5511:NaAl(SO
4765:Halides
4418:
3424:4465999
3396:Bibcode
3031:Bibcode
2969:Bibcode
2413:ammonia
2401:Belgian
2334:S + 2CO
2317:sulfide
2313:sulfate
2309:reduces
2209:barilla
2069:mummies
2000:+ NaHSO
1932:with CO
1908:Sodium
1812:sherbet
1716:→ CaCO
1338:by the
1274:what is
1272: (
1209:cations
1059:Warning
994:kJ/mol
990:−1044.4
978:(Δ
968:kJ/mol
964:−1130.7
918:entropy
713:Acidity
652:ammonia
640:acetone
628:alcohol
619:alkalis
506:Density
459:
430:..C()=O
275:PubChem
6288:(OH)CO
6188:HCOONa
6108:[Co(NO
6052:Fe(CN)
6040:Fe(CN)
6000:Zn(OH)
5689:NaHXeO
5523:NaAuCl
5476:Others
5224:NaHSeO
3973:Ca(HCO
3880:Mg(HCO
3618:
3591:
3534:
3509:
3447:
3422:
3414:
3388:Nature
3271:
3215:
2995:
2987:
2926:
2816:
2764:
2489:+ CaCl
2468:O + CO
2452:2NaHCO
2305:carbon
2284:+ 2HCl
2261:) and
2221:potash
2217:alkali
2194:potash
2121:Mining
2065:natron
1971:lignin
1965:(NaHSO
1912:(NaHCO
1885:dyeing
1784:lamian
1759:kansui
1750:gluten
1703:ions:
1660:silica
1630:, and
1364:natron
1328:potash
1269:verify
1266:
1207:Other
1194:anions
1192:Other
1176:(SDS)
992:
966:
935:
907:
725:10.33
705:
687:
669:
543:
536:
529:
522:
515:
477:
471:
423:SMILES
182:ChEMBL
70:Names
6441:COONa
6428:COONa
6341:AlNaO
6214:COONa
6201:COONa
6121:NaNSi
6116:]
5731:NaTcO
5723:NaTcO
5718:NaSCN
5710:NaReO
5705:NaOCN
5697:NaMnO
5669:NaHCO
5656:NaCoO
5651:NaCNO
5638:NaBiO
5542:NaAsF
5532:NaSbF
5491:NaAlO
5483:NaAlH
5464:NaSbO
5096:NaHSO
5088:NaHSO
5029:NaBrO
5021:NaBrO
5013:NaBrO
5008:NaBrO
5000:NaClO
4992:NaClO
4984:NaClO
4979:NaClO
4900:NaHTe
4886:NaSeH
4654:Th(CO
4333:Po(CO
4321:(BiO)
4234:CsHCO
3848:NaHCO
3747:LiHCO
3699:group
3420:S2CID
3352:(PDF)
3241:(PDF)
3177:(PDF)
3096:(PDF)
2955:(PDF)
2682:联合制碱法
2661:NaHCO
2525:ovens
2363:+ CaS
2175:Fucus
2126:Trona
2080:trona
1825:as a
1776:ramen
1643:borax
1634:(see
1632:paper
1624:glass
1615:(see
1606:salts
1404:), Na
1366:), Na
905:112.3
707:g/kg
388:InChI
288:10340
162:ChEBI
140:JSmol
6255:NaCH
6141:PdCl
6064:Fe(C
5916:O(UO
5904:PtCl
5892:IrCl
5739:NaVO
5646:NaCN
5582:NaBO
5577:(CN)
5573:NaBH
5565:NaBH
5444:HAsO
5299:NaPO
5263:NaNO
5255:NaNO
5045:NaIO
5037:NaIO
4942:NaNH
4872:NaSH
4858:NaOD
4853:NaOH
4800:NaAt
4790:NaBr
4785:NaCl
4777:NaHF
4510:EuCO
4356:RaCO
4313:PbCO
4293:HgCO
4242:BaCO
4194:CdCO
4174:PdCO
4143:SrCO
4103:ZnCO
4095:(OH)
4080:CuCO
4060:NiCO
4036:CoCO
4028:FeCO
4020:MnCO
3996:CrCO
3965:CaCO
3957:KHCO
3916:+SiO
3908:SiCO
3872:MgCO
3860:H(CO
3755:BeCO
3616:ISBN
3589:ISBN
3532:ISBN
3507:OCLC
3445:ISBN
3412:PMID
3359:2024
3335:2020
3309:2019
3269:ISBN
3213:ISBN
2993:PMID
2985:ISSN
2924:ISBN
2814:ISBN
2762:ISSN
2649:NaCl
2519:and
2508:and
2456:→ Na
2442:+ NH
2430:+ CO
2419:and
2355:→ Na
2311:the
2297:coal
2276:→ Na
2205:kelp
2198:kelp
2184:soda
1988:+ SO
1947:+ CO
1879:and
1858:base
1841:snus
1802:and
1678:(SiO
1662:(SiO
1658:for
1656:flux
1628:soap
1448:·10H
1374:·10H
1334:and
1303:and
1180:MSDS
1073:H319
819:Pca2
685:3.46
667:98.3
541:1.46
534:1.51
527:2.25
520:1.92
513:2.54
496:Odor
315:UNII
211:9916
6469:256
6465:164
6408:NaC
6349:NaC
6328:NO]
6312:NaC
6296:NaC
6280:NaC
6264:NaC
6259:COO
6247:NaO
6183:ONa
6170:ONa
6125:(CH
6096:SiF
6084:SiO
5960:TiO
5948:SiO
5880:MoO
5868:MnO
5839:GeO
5795:CrO
5681:XeO
5664:NaH
5556:TiF
5503:AlF
5456:AsO
5452:NaH
5432:AsO
5420:AsO
5315:HPO
5287:NaH
5236:TeO
5216:SeO
5204:SeO
4934:NaN
4824:NaO
4816:NaO
4795:NaI
4772:NaF
4707:No
4704:Md
4701:Fm
4698:Es
4695:Cf
4692:Bk
4689:Cm
4686:Am
4683:Pu
4680:Np
4665:Pa
4650:Ac
4647:**
4634:(CO
4618:(CO
4602:(CO
4586:(CO
4570:(CO
4554:(CO
4538:(CO
4522:(CO
4498:(CO
4490:Pm
4479:(CO
4463:(CO
4447:(CO
4431:(CO
4411:Og
4408:Ts
4405:Lv
4402:Mc
4399:Fl
4396:Nh
4393:Cn
4390:Rg
4387:Ds
4384:Mt
4381:Hs
4378:Bh
4375:Sg
4372:Db
4369:Rf
4366:Lr
4363:**
4352:Fr
4347:Rn
4344:At
4289:Au
4286:Pt
4283:Ir
4280:Os
4277:Re
4271:Ta
4268:Hf
4257:(CO
4216:Xe
4210:Te
4207:Sb
4204:Sn
4201:In
4170:Rh
4167:Ru
4164:Tc
4161:Mo
4158:Nb
4155:Zr
4125:Kr
4122:Br
4119:Se
4116:As
4113:Ge
4110:Ga
4048:(CO
4008:(CO
3989:Ti
3986:Sc
3939:Ar
3935:+Cl
3927:+SO
3896:(CO
3830:Ne
3815:+NO
3807:HCO
3787:(NH
3763:+BO
3729:He
3608:doi
3581:doi
3404:doi
3392:536
3205:doi
3155:doi
3039:doi
2977:doi
2848:633
2754:doi
2738:. H
2642:HCO
2630:HCO
2594:→ 2
2571:+ 4
2556:+ 2
2493:+ H
2481:2NH
2464:+ H
2434:+ H
2315:to
2168:or
2144:·2H
2136:HCO
2132:(Na
1992:+ H
1951:+ H
1898:CaO
1766:かん水
1738:lye
1720:(s)
1393:·7H
1026:GHS
957:298
933:135
926:298
703:0.5
697:in
679:in
661:in
357:EPA
278:CID
6499::
6479:Na
6473:68
6458:Na
6452:35
6448:18
6439:33
6435:17
6426:31
6422:15
6403:Na
6390:Na
6377:Na
6373:NO
6339:16
6324:Na
6314:10
6302:23
6298:12
6272:CO
6219:Na
6166:CH
6137:Na
6104:Na
6092:Na
6080:Na
6060:Na
6048:Na
6036:Na
6030:28
6026:10
6020:Na
6012:VO
6008:Na
5996:Na
5988:WO
5984:Na
5968:Na
5956:Na
5944:Na
5928:Na
5912:Na
5900:Na
5888:Na
5876:Na
5864:Na
5856:Na
5851:He
5847:Na
5835:Na
5829:10
5823:Cr
5819:Na
5807:Cr
5803:Na
5791:Na
5775:Na
5759:Na
5751:CO
5747:Na
5677:Na
5632:13
5622:Na
5606:Na
5590:Na
5552:Na
5499:Na
5440:Na
5428:Na
5416:Na
5396:Na
5380:Na
5374:10
5364:Na
5356:PO
5352:Na
5340:PS
5336:Na
5327:PO
5323:Na
5311:Na
5291:PO
5271:Na
5232:Na
5212:Na
5200:Na
5184:Na
5168:Na
5152:Na
5136:Na
5120:Na
5104:Na
5080:SO
5076:Na
5068:SO
5064:Na
4963:As
4959:Na
4950:Na
4925:Na
4909:Po
4905:Na
4895:Te
4891:Na
4881:Se
4877:Na
4863:Na
4841:Na
4832:Na
4673:CO
4669:UO
4630:Yb
4614:Tm
4598:Er
4582:Ho
4566:Dy
4550:Tb
4534:Gd
4518:Eu
4494:Sm
4475:Nd
4459:Pr
4443:Ce
4427:La
4423:*
4325:CO
4305:CO
4301:Tl
4274:W
4253:Lu
4249:*
4226:CO
4222:Cs
4213:I
4186:CO
4182:Ag
4152:Y
4135:CO
4131:Rb
4091:CO
4087:Cu
4085:,
4072:CO
4068:Cu
4044:Co
4004:Cr
3992:V
3949:CO
3923:P
3892:Al
3856:Na
3840:CO
3836:Na
3826:+F
3822:O
3803:NH
3795:CO
3775:+C
3739:CO
3735:Li
3716:CO
3614:.
3606:.
3587:.
3558:11
3556:.
3552:.
3519:^
3476:.
3418:.
3410:.
3402:.
3390:.
3325:.
3298:.
3283:^
3253:^
3243:.
3227:^
3211:.
3185:^
3151:36
3149:.
3145:.
3098:.
3063:,
3051:^
3037:.
3027:38
3025:.
3011:^
2991:.
2983:.
2975:.
2965:59
2963:.
2957:.
2938:^
2908:^
2892:.
2876:^
2856:^
2828:^
2812:.
2808::
2780:^
2760:.
2750:95
2748:.
2744:.
2742:O"
2734:CO
2716:^
2659:+
2657:Cl
2653:NH
2651:→
2647:+
2638:NH
2626:NH
2624:→
2616:+
2611:CO
2609:+
2604:NH
2596:NH
2587:+
2566:CO
2564:→
2551:CH
2527:.
2460:CO
2446:Cl
2423::
2411:,
2374:.
2359:CO
2347:Na
2344::
2326:SO
2322:Na
2319::
2307:,
2280:SO
2272:SO
2265::
2245:,
2200:.
2140:CO
2109:.
2103:pH
1984:CO
1980:Na
1943:CO
1939:Na
1936::
1928:CO
1894:pH
1869:pH
1833:,
1710:2−
1638:).
1626:,
1619:).
1548:10
1533:CO
1520:Na
1496:10
1481:CO
1468:Na
1444:CO
1412:·H
1408:CO
1397:O.
1389:CO
1370:CO
1346:.
1317:CO
1313:Na
1299:,
1158:50
1156:LD
1030::
949:(Δ
813:P2
722:)
715:(p
638:,
633:CS
621:,
455:CO
451:Na
6481:2
6477:2
6475:S
6471:O
6467:H
6463:C
6456:2
6454:O
6450:H
6446:C
6437:H
6433:C
6424:H
6420:C
6414:5
6412:H
6410:5
6401:9
6399:H
6397:4
6395:C
6388:5
6386:H
6384:6
6382:C
6375:4
6371:8
6369:H
6367:5
6365:C
6359:6
6357:O
6355:7
6353:H
6351:6
6343:4
6337:H
6335:6
6333:C
6326:2
6318:8
6316:H
6306:2
6304:O
6300:H
6290:2
6286:4
6284:H
6282:6
6274:2
6270:5
6268:H
6266:6
6257:3
6249:6
6245:5
6243:H
6241:4
6239:C
6233:6
6231:O
6229:4
6227:H
6225:4
6223:C
6221:2
6212:7
6210:H
6208:3
6206:C
6199:5
6197:H
6195:2
6193:C
6181:5
6179:H
6177:2
6175:C
6168:3
6143:4
6139:2
6131:6
6129:)
6127:3
6123:2
6114:6
6112:)
6110:2
6106:3
6098:6
6094:2
6086:4
6082:4
6074:3
6072:)
6070:4
6068:O
6066:2
6062:3
6054:6
6050:3
6042:6
6038:4
6028:O
6024:V
6022:6
6014:4
6010:3
6002:4
5998:2
5990:4
5986:2
5978:7
5976:O
5974:2
5972:U
5970:2
5962:3
5958:2
5950:3
5946:2
5938:6
5936:O
5934:4
5932:S
5930:2
5922:2
5920:)
5918:3
5914:2
5906:6
5902:2
5894:6
5890:3
5882:4
5878:2
5870:4
5866:2
5858:2
5849:2
5841:3
5837:2
5827:O
5825:3
5821:2
5813:7
5811:O
5809:2
5805:2
5797:4
5793:2
5785:5
5783:S
5781:3
5779:C
5777:2
5769:4
5767:O
5765:2
5763:C
5761:2
5753:3
5749:2
5741:3
5733:4
5725:3
5712:4
5699:4
5691:4
5683:6
5679:4
5671:3
5658:2
5640:3
5630:O
5628:8
5626:B
5624:2
5616:9
5614:O
5612:2
5610:B
5608:2
5600:7
5598:O
5596:4
5594:B
5592:2
5584:2
5575:3
5567:4
5558:6
5554:2
5544:6
5534:6
5525:4
5517:2
5515:)
5513:4
5505:6
5501:3
5493:2
5485:4
5466:3
5458:4
5454:2
5446:4
5442:2
5434:4
5430:3
5422:3
5418:3
5410:7
5408:O
5406:2
5404:P
5402:2
5400:H
5398:2
5390:7
5388:O
5386:2
5384:P
5382:4
5372:O
5370:3
5368:P
5366:5
5358:4
5354:3
5346:2
5344:O
5342:2
5338:3
5331:F
5329:3
5325:2
5317:3
5313:2
5305:2
5303:H
5301:2
5293:4
5289:2
5281:2
5279:O
5277:2
5275:N
5273:2
5265:3
5257:2
5238:3
5234:2
5226:3
5218:4
5214:2
5206:3
5202:2
5194:8
5192:O
5190:2
5188:S
5186:2
5178:7
5176:O
5174:2
5172:S
5170:2
5162:6
5160:O
5158:2
5156:S
5154:2
5146:5
5144:O
5142:2
5140:S
5138:2
5130:4
5128:O
5126:2
5124:S
5122:2
5114:3
5112:O
5110:2
5108:S
5106:2
5098:4
5090:3
5082:4
5078:2
5070:3
5066:2
5047:4
5039:3
5031:4
5023:3
5015:2
5002:4
4994:3
4986:2
4961:3
4954:P
4952:3
4944:2
4936:3
4929:N
4927:3
4907:2
4893:2
4879:2
4867:S
4865:2
4847:2
4845:O
4843:2
4836:O
4834:2
4826:2
4818:3
4779:2
4743:e
4736:t
4729:v
4675:3
4671:2
4660:2
4658:)
4656:3
4640:3
4638:)
4636:3
4632:2
4624:3
4622:)
4620:3
4616:2
4608:3
4606:)
4604:3
4600:2
4592:3
4590:)
4588:3
4584:2
4576:3
4574:)
4572:3
4568:2
4560:3
4558:)
4556:3
4552:2
4544:3
4542:)
4540:3
4536:2
4528:3
4526:)
4524:3
4520:2
4515:,
4512:3
4504:3
4502:)
4500:3
4496:2
4485:3
4483:)
4481:3
4477:2
4469:3
4467:)
4465:3
4461:2
4453:3
4451:)
4449:3
4445:2
4437:3
4435:)
4433:3
4429:2
4358:3
4339:2
4337:)
4335:3
4327:3
4323:2
4315:3
4307:3
4303:2
4295:3
4263:3
4261:)
4259:3
4255:2
4244:3
4236:3
4231:,
4228:3
4224:2
4196:3
4188:3
4184:2
4176:3
4145:3
4137:3
4133:2
4105:3
4097:2
4093:3
4089:2
4082:3
4077:,
4074:3
4070:2
4062:3
4054:3
4052:)
4050:3
4046:2
4041:,
4038:3
4030:3
4022:3
4014:3
4012:)
4010:3
4006:2
4001:,
3998:3
3979:2
3977:)
3975:3
3970:,
3967:3
3959:3
3954:,
3951:3
3947:2
3945:K
3929:4
3918:4
3913:,
3910:4
3902:3
3900:)
3898:3
3894:2
3886:2
3884:)
3882:3
3877:,
3874:3
3866:2
3864:)
3862:3
3858:3
3853:,
3850:3
3845:,
3842:3
3838:2
3817:3
3812:,
3809:3
3805:4
3800:,
3797:3
3793:2
3791:)
3789:4
3781:4
3779:O
3777:2
3765:3
3757:3
3749:3
3744:,
3741:3
3737:2
3718:3
3714:2
3712:H
3688:e
3681:t
3674:v
3624:.
3610::
3597:.
3583::
3538:.
3513:.
3487:.
3453:.
3426:.
3406::
3398::
3375:.
3361:.
3337:.
3311:.
3277:.
3247:.
3221:.
3207::
3179:.
3161:.
3157::
3130:.
3109:.
3081:.
3045:.
3041::
3033::
3005:.
2979::
2971::
2932:.
2902:.
2850:.
2822:.
2774:.
2756::
2740:2
2736:3
2732:2
2680:(
2663:3
2655:4
2644:3
2640:4
2632:3
2628:4
2622:O
2620:2
2618:H
2613:2
2606:3
2598:3
2591:2
2589:N
2584:2
2582:H
2580:3
2575:2
2573:H
2568:2
2562:O
2560:2
2558:H
2553:4
2497:O
2495:2
2491:2
2487:3
2483:4
2470:2
2466:2
2462:3
2458:2
2454:3
2444:4
2440:3
2436:2
2432:2
2428:3
2361:3
2357:2
2353:3
2349:2
2336:2
2332:2
2328:4
2324:2
2291:(
2282:4
2278:2
2274:4
2270:2
2257:(
2219:"
2146:2
2142:3
2138:3
2134:3
2002:3
1998:3
1994:2
1990:2
1986:3
1982:2
1967:3
1957:3
1953:2
1949:2
1945:3
1941:2
1934:2
1930:3
1926:2
1922:3
1918:3
1914:3
1769:)
1763:(
1718:3
1713:3
1680:2
1664:2
1645:.
1565:O
1557:2
1553:H
1537:3
1524:2
1513:O
1505:2
1501:H
1493:+
1485:3
1472:2
1450:2
1446:3
1442:2
1427:.
1420:.
1414:2
1410:3
1406:2
1395:2
1391:3
1387:2
1376:2
1372:3
1368:2
1319:3
1315:2
1264:N
1164:)
1160:(
1136:0
1129:0
1122:2
985:)
983:G
980:f
959:)
954:H
951:f
928:)
923:S
921:(
900:)
898:C
896:(
863:c
859:b
855:a
821:1
815:1
754:)
752:D
749:n
747:(
720:a
717:K
635:2
457:3
453:2
359:)
355:(
142:)
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.