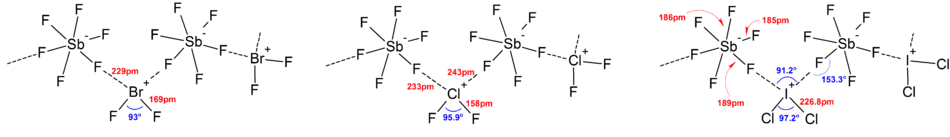699:
731:
1242:
691:
767:
1398:
1373:
Linear or nearly-linear triatomic polyhalides have weaker and longer bonds compared with that in the corresponding diatomic interhalogen or halogen, consistent with the additional repulsion between atoms as the halide ion is added to the neutral molecule. Another model involving the use of
1316:
The bonding in polyhalogen ions mostly invoke the predominant use of p-orbitals. Significant d-orbital participation in the bonding is improbable as much promotional energy will be required, while scant s-orbital participation is expected in iodine-containing species due to the
4332:
The anions are less reactive compared to the cations, and are generally weaker oxidants than their parent interhalogens. They are less reactive towards organic compounds, and some salts are of quite high thermal stability. Salts containing polyhalogen anions of the type
1447:
Even though they have a reduced bond order, all three halogen atoms are tightly bound. The fluorine–fluorine bond of trifluoride, with bond order 0.5, has a bond-strength is 30 kcal/mol, only 8 kcal/mol less than the fluorine–fluorine bond in
3991:
The heteropolyhalogen cations are explosively reactive oxidants, and the cations often have higher reactivity than their parent interhalogens and decompose by reductive pathways. As expected from the highest oxidation state of +7 in
1307:
units, which reflects the origin of the polyiodide. In the solid states, the polyiodides can interact with each other to form chains, rings, or even complicated two-dimensional and three-dimensional networks.
1695:
Usually the first method is employed for preparing heteropolyhalogen cations, and the second one is applicable to both. The oxidative process is useful in the preparation of the cations
1288:
ions have much more complicated structures. Discrete polyiodides usually have a linear sequence of iodine atoms and iodide ions, and are described in terms of association between
810:. The polyhalogen ions always have the heaviest and least electronegative halogen present in the ion as the central atom, making the ion asymmetric in some cases. For example,
38:
only. The ions can be classified into two classes, isopolyhalogen ions which contain one type of halogen only, and heteropolyhalogen ions with more than one type of halogen.
46:
Numerous polyhalogen ions have been found, with their salts isolated in the solid state and structurally characterized. The following tables summarize the known species.
857:. More deviations from the ideal VSEPR model were found in the solid state structures due to strong cation-anion interactions, which also complicates interpretation of
3578:
The polyhalogen cations are strong oxidizing agents, as indicated by the fact that they can only be prepared in oxidative liquids as a solvent, such as
3800:
3780:
Most polyhalogen ions are intensely colored, with deepened color as the atomic weight of the constituent element increases. The well-known
4375:
halogen, so that the monohalide has the highest lattice energy. An interhalogen is usually formed as the other product. The salt
698:
4691:
1632:
By an oxidative process, in which the halogen or interhalogen is reacted with an oxidizer and a Lewis acid to give the cation:
4551:
4506:
1391:
3730:
For polyhalogen anions with the same number of atoms, the more stable ones are those with a heavier halogen at the center,
1325:. However, no bonding model has been capable of reproducing such wide range of bond lengths and angles observed so far.
4591:
854:
4619:
4464:
3772:
Heteropolyhalogen ions with a coordination number larger than or equal to four can only exist with fluoride ligands.
853:, they have a regular octahedral arrangement of fluoride ligands instead of a distorted one due to the presence of a
829:
In general, the structures of most heteropolyhalogen ions and lower isopolyhalogen ions were in agreement with the
3734:
ions are also more stable than asymmetric ones. therefore the stability of the anions decrease in the order:
4154:
3625:
salts (X = Br, I) are thermodynamically quite stable. However, their stability in solution depends on the
3514:
The higher polyiodides were formed upon crystallization of solutions containing various concentrations of
1193:
is distorted octahedral as the stereochemical inert-pair effect is not significant in the chlorine atom.
4635:
Sonnenberg, Karsten; Mann, Lisa; Redeker, Frenio A.; Schmidt, Benjamin; Riedel, Sebastian (2020-02-04).
2878:
The preparation of some individual species are briefly summarized in the table below with equations:
1802:
The preparation of some individual species are briefly summarized in the table below with equations:
1322:
3649:
1461:
1428:
compared with 384 cm in ICl), which suggests a bond order of about 0.5 for each I–Cl bonds in
858:
833:. However, there were exceptional cases. For example, when the central atom is heavy and has seven
4016:, these species are extremely strong oxidizing agents, demonstrated by the reactions shown below:
861:
data. In all known structures of the polyhalogen anion salts, the anions make very close contact,
4158:
197:
3803:
tend to be colorless or pale yellow, other heteropolyhalogen ions are orange, red or deep purple
3690:
1673:
1363:
gets higher, consequently the interatomic distances in the molecular ion is less than those in
730:
4543:
3681:
2769:
1375:
1241:
807:
4179:
The dimerization can be attributed to the overlapping of the half-filled π* orbitals in two
690:
4093:
3638:
223:
is only known from its electronic band spectrum obtained in a low-pressure discharge tube.
8:
4455:
King, R. Bruce (2005). "Chlorine, Bromine, Iodine, & Astatine: Inorganic
Chemistry".
1962:
1436:, consistent with the interpretation using the resonance theory. Other triatomic species
1413:
1329:
4726:
4672:
4497:
Housecroft, Catherine E.; Sharpe, Alan G. (2008). "Chapter 17: The group 17 elements".
4081:
3731:
2996:
803:
4582:
Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999).
4774:
4718:
4676:
4664:
4656:
4615:
4587:
4547:
4502:
4460:
4418:
4274:
1591:
1318:
4730:
3324:
the reactants were mixed at 242 K, then warmed to 298 K for the reaction to proceed
4710:
4648:
4535:
3541:
1387:
766:
4372:
3699:
1170:-type species known to have the rare pentagonal planar geometry, the other being
799:
3572:
3000:
3011:
does not exist in solution and is only formed when the salt crystallizes out.
2787:
For polyhalogen anions, there are two general preparation strategies as well:
2768:/HF system. It is an even more powerful oxidizing and fluorinating agent than
4768:
4660:
1397:
1352:
1404:
Evidence supporting this theory comes from the bond lengths (255 pm in
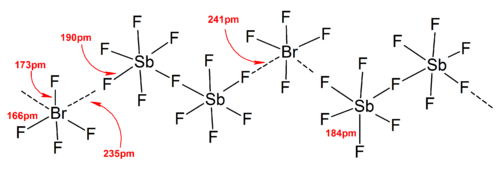
865:
halogen bridges, with the counter-cations. For example, in the solid state,
4722:
4668:
4652:
4413:
4367:= {3, 5, 7, 9...}, tend to dissociate into simple monohalide salts between
4132:
3506:
3441:
1563:
1465:
4636:
4143:
3979:
3571:) can help stabilize the polyhalogen ions formed in the solid state from
3533:
crystallizes when a saturated solution containing appropriate amounts of
1417:
830:
3924:
exists as greenish-black needles, but appears brown-red in thin sections
4423:
4408:
4034:
2792:
1579:
1567:
1449:
1348:
1285:
1279:
834:
17:
4714:
3582:. The most oxidizing and therefore most unstable ones are the species
4428:
3626:
1420:(267 and 222 cm for symmetric and asymmetric stretching in
4131:
reversibly dimerizes at 193 K, and is observed as the blue color of
1558:
There are two general strategies for preparing polyhalogen cations:
3207:
1660:
1587:
1575:
4637:"Polyhalogen and Polyinterhalogen Anions from Fluorine to Iodine"
4277:
have met with failure, because the following reactions occurred:
3793:
3559:
1583:
1469:
901:
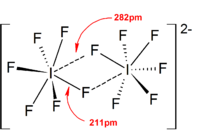
dimers. Significant cation-anion interactions were also found in
35:
4610:
Wiberg, Egon; Wiberg, Nils; Holleman, Arnold
Frederick (2001).
3781:
2856:
1328:
As expected from the fact that an electron is removed from the
27:
4581:
4696:
So
Special? A Breathing-Orbital Valence Bond ab Initio Study"
4057:
3579:
1590:) either in an inert or oxidizing solvent (such as anhydrous
1571:
31:
4634:
3796:
helix. Some colors of the common species were listed below:
3078:
in 1,2-dichloroethane or liquid sulfur dioxide, with excess
4092:
is unstable in solution and disproportionate completely in
798:
Most of the structures of the ions have been determined by
873:
is not regularly octahedral, as solid state structure of
4080:
Polyhalogen cations with lower oxidation states tend to
3784:-iodine complex has a deep blue color due to the linear
4399:
are thermally unstable and can explode even at −31 °C.
2123:
in anhydrous HF, at a temperature below 193 K (-80 °C)
1460:
The formation of polyhalogen ions can be viewed as the
938:
General structures of selected heteropolyhalogen ions
3982:
have very dark colors, either dark brown or dark blue
3575:
considerations, as the packing efficiency increases.
3557:
In general, a large counter cation or anion (such as
1594:) or without one, to give a heteropolyhalogen cation.
4609:
2737:In this reaction, the active oxidizing species is
4534:
4766:
4496:
702:Solid state structures of the polyhalogen ions
4577:
4575:
4573:
4571:
4569:
4567:
4565:
4563:
4142:dramatically shifts to the red-brown color of
2791:By reacting an interhalogen or halogen with a
4690:Braïda, Benoît; Hiberty, Philippe C. (2004).
4689:
3814:are wine red to bright orange; while that of
4605:
4603:
4161:when the solution is cooled to below 193 K:
4755:Handbook of Preparative Inorganic Chemistry
4560:
4530:
4528:
4526:
4524:
4522:
4520:
4518:
4492:
4490:
4488:
4486:
4484:
4482:
4480:
4478:
4476:
3934:is black, if its existence in the compound
4757:(2nd ed.). New York: Academic Press.
4600:
4450:
4448:
4446:
4444:
4391:decomposes at about 100 °C, and salts of
694:Structures of some isopolyhalogen cations
4748:
4746:
4744:
4742:
4740:
4515:
4473:
1240:
765:
729:
697:
689:
4641:Angewandte Chemie International Edition
1726:respectively, has never been isolated:
4767:
4752:
4501:(3rd ed.). Pearson. p. 547.
4441:
3986:
1807:Synthesis of some polyhalogen cations
1553:
1230:-type structure, analogous to that of
4737:
3680:when weaker fluoride acceptors, like
3198:], [AtICl], [AtIBr], [AtI
2882:Synthesis of some polyhalogen anions
2782:
1663:acceptor) itself acts as an oxidant:
1273:
542:], [AtICl], [AtIBr], [AtI
4614:. Academic Press. pp. 419–420.
4459:(2nd ed.). Wiley. p. 747.
4454:
3657:= −20.65), but disproportionates to
1874:in anhydrous HF at low temperatures
526:], [IBrF], [IBrCl], [IBr
4692:"What Makes the Trifluoride Anion F
4457:Encyclopedia of Inorganic Chemistry
3525:. For instance, the monohydrate of
2005:at a temperature of 195 K (-78 °C)
13:
4217:, but decomposes at 195 K to give
1659:In some cases the Lewis acid (the
1396:
14:
4786:
1711:, as their parent interhalogens,
239:is possible but still uncertain.
3822:are dark brown to purplish black
855:stereochemically inert lone pair
2893:Additional conditions required
1818:Additional conditions required
41:
4683:
4628:
1444:can be similarly interpreted.
1:
4434:
4209:is structurally analogous to
3547:
2354:at a temperature below 197 K
4584:Advanced Inorganic Chemistry
4316:] + 2 NOF → [NO][AsF
3912:is green or black, the salt
3552:
1562:By reacting the appropriate
1455:
685:
538:Br], [AtBrCl], [AtBr
7:
4402:
4155:paramagnetic susceptibility
3946:has been firmly established
2373:+ 2 Au → 3 BrF + 2 [BrF
10:
4791:
4290:] + NOF → [NO][PtF
4153:, together with a drop in
3598:(X = Cl, Br), followed by
3212:(X = I, Br, Cl; Y = I, Br)
2890:Relevant chemical equation
2795:, most likely a fluoride:
2614:
1815:Relevant chemical equation
1311:
1277:
1111:
1078:
245:Heteropolyhalogen cations
142:
59:
26:are a group of polyatomic
15:
4540:Chemistry of the Elements
4538:; Earnshaw, Alan (1997).
1416:(g)) and bond stretching
1321:, suggested by data from
1245:Solid state structure of
943:Linear (or almost linear)
859:vibrational spectroscopic
734:Solid state structure of
497:Heteropolyhalogen anions
4753:Brauer, G., ed. (1963).
3775:
3129:+ 2 CsCl → 2 Cs[BrCl
1570:(such as the halides of
1053:Disphenoidal (or seesaw)
274:], [IBrCl], [IBr
16:Not to be confused with
4586:(6th ed.). Wiley.
4159:electrical conductivity
4102:mixture even at 197 K:
3702:, are added instead of
2855:By oxidation of simple
1452:whose bond order is 1.
198:charge-transfer complex
196:at low temperatures, a
51:Isopolyhalogen cations
4653:10.1002/anie.201903197
3892:is dark brown to black
3629:solvent. For example,
3447:P]Br + 3 IBr → [Ph
3099:ClF + CsF → Cs[ClF
1401:
1323:Mössbauer spectroscopy
1270:
889:reveals loosely bound
795:
763:
727:
695:
346:Isopolyhalogen anions
4544:Butterworth-Heinemann
4536:Greenwood, Norman N.
4068:] → [RnF][SbF
3617:The stability of the
2195:+ 3 [XeF][AsF
1400:
1386:can be viewed as the
1378:exists, for example,
1244:
808:X-ray crystallography
769:
733:
701:
693:
4320:] + [NO][BrF
4243:Attempts to prepare
3792:ions present in the
3639:fluoroantimonic acid
1918:at room temperature
1133:Square antiprismatic
4709:(45): 14890–14898.
4612:Inorganic Chemistry
4499:Inorganic Chemistry
3987:Chemical properties
2883:
1808:
1785:+ [KrF][AsF
1554:Polyhalogen cations
1412:and 232 pm in
1330:antibonding orbital
939:
818:has a structure of
640:Heptaatomic species
552:Pentaatomic species
498:
405:Heptaatomic species
391:Pentaatomic species
373:Tetraatomic species
347:
322:Heptaatomic species
292:Pentaatomic species
246:
139:Heptaatomic species
121:Pentaatomic species
103:Tetraatomic species
52:
3718:] + 5 F → 9 [I
3233:→ [NO][ClF
3210:Y + X → [AtXY]
2997:1,2-dichloroethane
2881:
2783:Polyhalogen anions
2745:, which is formed
1806:
1402:
1274:Higher polyiodides
1271:
1161:is one of the two
937:
804:Raman spectroscopy
796:
764:
728:
696:
670:Nonaatomic species
626:Hexaatomic species
496:
419:Octaatomic species
345:
244:
184:can only exist as
50:
4715:10.1021/ja046443a
4647:(14): 5464–5493.
4553:978-0-08-037941-8
4508:978-0-13-175553-6
4419:Inorganic polymer
3512:
3511:
3415:+ CsF → Cs[IF
2729:
2728:
2672:[KrF][AsF
1462:self-dissociation
1390:of the following
1319:inert-pair effect
1146:
1145:
1075:Pentagonal planar
782:dimer present in
770:Structure of the
683:
682:
502:Triatomic species
494:
493:
351:Triatomic species
343:
342:
250:Triatomic species
231:The existence of
169:
168:
81:Triatomic species
4782:
4759:
4758:
4750:
4735:
4734:
4703:J. Am. Chem. Soc
4700:
4687:
4681:
4680:
4632:
4626:
4625:
4607:
4598:
4597:
4579:
4558:
4557:
4542:(2nd ed.).
4532:
4513:
4512:
4494:
4471:
4470:
4452:
4398:
4390:
4370:
4354:
4328:
4302:
4272:
4264:
4257:by fluorinating
4256:
4249:
4239:
4231:
4223:
4216:
4208:
4196:
4186:
4175:
4152:
4141:
4130:
4120:
4100:
4091:
4082:disproportionate
4076:
4053:
4015:
4007:
3999:
3976:is golden-yellow
3975:
3965:
3955:
3945:
3933:
3923:
3911:
3901:
3891:
3881:
3871:
3861:
3851:
3841:
3831:
3821:
3813:
3791:
3768:
3759:] > [BrCl
3755:Br] > [Br
3726:
3708:
3696:
3687:
3679:
3672:
3664:
3647:
3636:
3624:
3613:
3605:
3597:
3589:
3570:
3562:
3539:
3532:
3524:
3517:
3502:
3473:
3460:
3437:
3420:
3407:
3394:
3361:
3348:
3335:
3321:
3292:
3280:
3271:
3251:
3238:
3225:
3211:
3203:
3186:
3173:
3160:
3147:
3134:
3117:
3104:
3095:
3084:
3075:
3022:
3010:
2992:
2939:
2925:
2912:
2884:
2880:
2872:
2850:
2837:
2775:
2767:
2760:
2744:
2736:
2735:
2723:
2702:
2689:
2668:
2655:
2611:
2598:
2573:
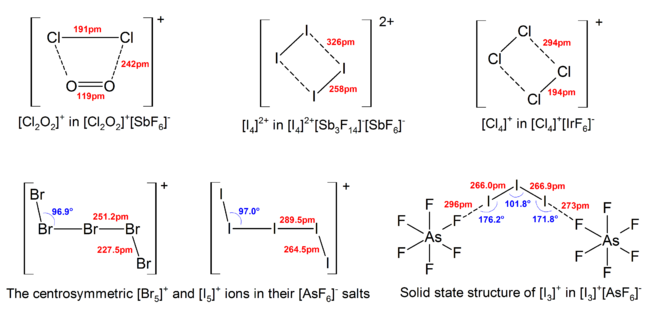
2560:
2539:
2526:
2505:
2492:
2471:
2458:
2437:
2424:
2403:
2391:
2382:
2365:
2351:
2334:
2321:
2300:
2287:
2258:
2245:
2224:
2211:
2187:
2176:
2167:
2162:
2161:
2158:
2134:
2120:
2099:
2086:
2057:
2044:
2016:
2002:
1981:
1969:
1958:
1929:
1914:
1885:
1871:
1841:
1829:
1809:
1805:
1798:
1777:
1748:
1725:
1710:
1691:
1655:
1627:
1549:
1532:
1515:
1498:
1464:of their parent
1443:
1435:
1427:
1411:
1388:resonance hybrid
1385:
1376:resonance theory
1369:
1362:
1346:
1338:
1306:
1298:
1294:
1268:
1252:
1237:
1221:
1209:
1192:
1185:
1184:
1177:
1169:
1160:
1153:
1152:
1142:
1128:
1109:
1087:
1070:
1048:
1022:
1021:Br], [IBrCl]
980:
940:
936:
932:
900:
888:
872:
852:
844:
825:
821:
817:
793:
781:
761:
745:
725:
717:
679:
665:
635:
621:
547:
499:
495:
490:
432:
414:
400:
386:
368:
348:
344:
339:
317:
287:
247:
243:
238:
230:
229:
222:
214:
206:
195:
183:
176:
175:
165:
151:
134:
116:
98:
76:
56:Diatomic species
53:
49:
24:Polyhalogen ions
4790:
4789:
4785:
4784:
4783:
4781:
4780:
4779:
4765:
4764:
4763:
4762:
4751:
4738:
4698:
4695:
4688:
4684:
4633:
4629:
4622:
4608:
4601:
4594:
4580:
4561:
4554:
4546:. p. 835.
4533:
4516:
4509:
4495:
4474:
4467:
4453:
4442:
4437:
4405:
4396:
4392:
4388:
4384:
4380:
4376:
4373:electronegative
4368:
4352:
4346:
4340:
4334:
4327:
4323:
4319:
4315:
4311:
4307:
4301:
4297:
4293:
4289:
4285:
4281:
4270:
4266:
4262:
4258:
4255:
4251:
4248:
4244:
4237:
4233:
4229:
4225:
4224:, and salts of
4222:
4218:
4214:
4210:
4206:
4202:
4198:
4194:
4190:
4184:
4180:
4173:
4169:
4165:
4150:
4146:
4139:
4135:
4128:
4124:
4118:
4114:
4110:
4106:
4099:
4095:
4089:
4085:
4084:. For example,
4075:
4071:
4067:
4063:
4056:
4052:
4048:
4044:
4038:
4032:
4028:
4024:
4020:
4013:
4009:
4005:
4001:
3997:
3993:
3989:
3973:
3969:
3963:
3959:
3953:
3949:
3943:
3939:
3935:
3931:
3927:
3921:
3917:
3913:
3909:
3905:
3902:is red to brown
3899:
3895:
3889:
3885:
3879:
3875:
3869:
3865:
3859:
3855:
3849:
3845:
3839:
3835:
3829:
3825:
3819:
3815:
3811:
3807:
3789:
3785:
3778:
3766:
3762:
3758:
3754:
3750:
3747:] > [ICl
3746:
3743:] > [IBr
3742:
3738:
3725:
3721:
3717:
3713:
3707:
3703:
3695:
3691:
3686:
3682:
3678:
3674:
3670:
3666:
3662:
3658:
3655:
3646:
3642:
3641:(HF with 0.2 N
3634:
3630:
3622:
3618:
3611:
3607:
3603:
3599:
3595:
3591:
3587:
3583:
3568:
3564:
3558:
3555:
3550:
3538:
3534:
3530:
3526:
3523:
3519:
3515:
3500:
3496:
3492:
3488:
3484:
3480:
3476:
3471:
3467:
3458:
3454:
3450:
3446:
3440:
3435:
3431:
3427:
3418:
3414:
3410:
3405:
3401:
3392:
3388:
3384:
3380:
3376:
3372:
3368:
3364:
3359:
3355:
3346:
3342:
3338:
3333:
3329:
3319:
3315:
3311:
3307:
3303:
3299:
3295:
3290:
3286:
3279:
3275:
3270:
3266:
3262:
3258:
3254:
3249:
3245:
3236:
3232:
3228:
3223:
3219:
3206:
3201:
3197:
3193:
3184:
3180:
3176:
3171:
3167:
3158:
3154:
3150:
3145:
3141:
3132:
3128:
3124:
3120:
3115:
3111:
3102:
3098:
3093:
3089:
3083:
3079:
3073:
3069:
3065:
3061:
3057:
3053:
3050:N]Br → [(CH
3049:
3045:
3041:
3037:
3033:
3029:
3025:
3020:
3016:
3008:
3004:
2990:
2986:
2982:
2978:
2974:
2970:
2967:N]Br → [(CH
2966:
2962:
2958:
2954:
2950:
2946:
2942:
2937:
2933:
2926:(X = Cl, Br, I)
2923:
2919:
2915:
2910:
2906:
2902:
2898:
2870:
2866:
2862:
2848:
2844:
2840:
2835:
2828:
2824:
2820:
2816:
2810:
2806:
2802:
2798:
2785:
2780:
2779:
2774:
2770:
2766:
2762:
2758:
2754:
2750:
2742:
2738:
2733:
2732:
2721:
2717:
2713:
2709:
2705:
2700:
2696:
2687:
2683:
2679:
2675:
2671:
2666:
2662:
2653:
2649:
2645:
2641:
2637:
2633:
2629:
2625:
2621:
2617:
2609:
2605:
2596:
2592:
2588:
2584:
2580:
2576:
2571:
2567:
2558:
2554:
2550:
2546:
2542:
2537:
2533:
2524:
2520:
2516:
2512:
2508:
2503:
2499:
2490:
2486:
2482:
2478:
2474:
2469:
2465:
2456:
2452:
2448:
2444:
2440:
2435:
2431:
2422:
2418:
2414:
2410:
2406:
2401:
2397:
2390:
2386:
2380:
2376:
2372:
2368:
2363:
2359:
2349:
2345:
2341:
2337:
2332:
2328:
2319:
2315:
2311:
2307:
2303:
2298:
2294:
2285:
2281:
2277:
2273:
2269:
2265:
2261:
2256:
2252:
2243:
2239:
2235:
2231:
2227:
2222:
2218:
2210:
2206:
2202:
2198:
2194:
2190:
2185:
2181:
2175:
2171:
2166:
2159:
2156:
2155:
2153:
2149:
2145:
2141:
2137:
2132:
2128:
2118:
2114:
2110:
2106:
2102:
2097:
2093:
2084:
2080:
2076:
2072:
2068:
2064:
2060:
2055:
2051:
2043:
2039:
2035:
2031:
2027:
2023:
2019:
2014:
2010:
2000:
1996:
1992:
1988:
1984:
1979:
1975:
1967:
1963:
1956:
1952:
1948:
1944:
1940:
1936:
1932:
1927:
1923:
1912:
1908:
1904:
1900:
1896:
1892:
1888:
1883:
1879:
1869:
1865:
1861:
1857:
1853:
1849:
1845:
1839:
1835:
1831:
1827:
1823:
1796:
1792:
1788:
1784:
1780:
1775:
1771:
1767:
1763:
1759:
1755:
1751:
1746:
1742:
1738:
1734:
1730:
1724:
1720:
1716:
1712:
1708:
1704:
1700:
1696:
1690:
1686:
1682:
1677:
1671:
1667:
1653:
1649:
1645:
1641:
1637:
1625:
1618:
1611:
1605:
1599:
1556:
1547:
1543:
1539:
1535:
1530:
1526:
1522:
1518:
1513:
1509:
1505:
1501:
1496:
1489:
1482:
1476:
1458:
1441:
1437:
1433:
1429:
1425:
1421:
1409:
1405:
1392:canonical forms
1383:
1379:
1368:
1364:
1360:
1356:
1351:as well as the
1344:
1340:
1337:
1333:
1314:
1304:
1300:
1296:
1293:
1289:
1282:
1276:
1266:
1262:
1258:
1254:
1250:
1246:
1235:
1231:
1219:
1215:
1211:
1207:
1203:
1199:
1196:
1195:
1190:
1186:
1182:
1181:
1175:
1171:
1168:
1162:
1158:
1154:
1150:
1149:
1140:
1136:
1126:
1122:
1118:
1114:
1107:
1103:
1099:
1095:
1085:
1081:
1068:
1064:
1060:
1056:
1046:
1042:
1038:
1034:
1030:
1020:
1016:
1012:
1008:
1004:
1000:
996:
992:
988:
978:
974:
970:
966:
962:
958:
954:
950:
946:
930:
926:
922:
918:
914:
910:
906:
902:
898:
894:
890:
886:
882:
878:
874:
870:
866:
850:
846:
842:
838:
823:
819:
815:
811:
800:IR spectroscopy
791:
787:
783:
779:
775:
771:
759:
755:
751:
747:
743:
739:
735:
723:
719:
715:
711:
707:
703:
688:
677:
673:
663:
659:
655:
651:
647:
643:
633:
629:
619:
615:
611:
607:
603:
599:
595:
591:
587:
583:
579:
575:
571:
567:
563:
559:
555:
545:
541:
537:
533:
529:
525:
521:
517:
513:
509:
505:
488:
484:
480:
476:
472:
468:
464:
460:
456:
452:
448:
444:
440:
430:
426:
422:
412:
408:
398:
394:
384:
380:
376:
366:
362:
358:
354:
337:
333:
329:
325:
315:
311:
307:
303:
299:
295:
285:
281:
277:
273:
269:
265:
261:
257:
253:
242:
241:
236:
232:
227:
226:
220:
216:
212:
208:
205:
201:
193:
189:
185:
181:
177:
173:
172:
163:
159:
149:
145:
132:
128:
124:
114:
110:
106:
96:
92:
88:
84:
74:
70:
66:
62:
44:
21:
12:
11:
5:
4788:
4778:
4777:
4761:
4760:
4736:
4693:
4682:
4627:
4620:
4599:
4593:978-0471199571
4592:
4559:
4552:
4514:
4507:
4472:
4465:
4439:
4438:
4436:
4433:
4432:
4431:
4426:
4421:
4416:
4411:
4404:
4401:
4394:
4386:
4382:
4378:
4348:
4342:
4336:
4330:
4329:
4325:
4321:
4317:
4313:
4309:
4304:
4303:
4299:
4295:
4291:
4287:
4283:
4268:
4260:
4253:
4246:
4235:
4227:
4220:
4212:
4204:
4200:
4192:
4182:
4177:
4176:
4171:
4167:
4148:
4137:
4126:
4122:
4121:
4116:
4112:
4108:
4097:
4087:
4078:
4077:
4073:
4069:
4065:
4061:
4054:
4050:
4046:
4042:
4036:
4030:
4026:
4022:
4011:
4003:
3995:
3988:
3985:
3984:
3983:
3977:
3971:
3967:
3966:is scarlet red
3961:
3957:
3951:
3947:
3941:
3937:
3929:
3925:
3919:
3915:
3907:
3903:
3897:
3893:
3887:
3883:
3882:is bright blue
3877:
3873:
3867:
3863:
3857:
3853:
3847:
3843:
3837:
3833:
3827:
3823:
3817:
3809:
3804:
3787:
3777:
3774:
3770:
3769:
3764:
3763:] > [Br
3760:
3756:
3752:
3748:
3744:
3740:
3728:
3727:
3723:
3719:
3715:
3705:
3693:
3684:
3676:
3668:
3660:
3653:
3644:
3632:
3620:
3609:
3601:
3593:
3585:
3573:lattice energy
3566:
3554:
3551:
3549:
3546:
3536:
3528:
3521:
3510:
3509:
3503:
3498:
3494:
3490:
3489:N]F → [(CH
3486:
3482:
3478:
3474:
3469:
3464:
3463:
3461:
3456:
3452:
3448:
3444:
3438:
3433:
3429:
3424:
3423:
3421:
3416:
3412:
3408:
3403:
3398:
3397:
3395:
3390:
3386:
3382:
3378:
3377:N]F → [(CH
3374:
3370:
3366:
3362:
3357:
3352:
3351:
3349:
3344:
3340:
3336:
3331:
3326:
3325:
3322:
3317:
3313:
3309:
3308:N]I → [(CH
3305:
3301:
3297:
3293:
3288:
3283:
3282:
3277:
3272:
3268:
3264:
3260:
3256:
3252:
3247:
3242:
3241:
3239:
3234:
3230:
3226:
3221:
3216:
3215:
3213:
3204:
3199:
3195:
3190:
3189:
3187:
3182:
3178:
3174:
3169:
3164:
3163:
3161:
3156:
3152:
3148:
3143:
3138:
3137:
3135:
3130:
3126:
3122:
3118:
3113:
3108:
3107:
3105:
3100:
3096:
3091:
3086:
3085:
3081:
3076:
3071:
3067:
3063:
3059:
3055:
3051:
3047:
3043:
3039:
3035:
3031:
3027:
3023:
3018:
3013:
3012:
3006:
3001:sulfur dioxide
2993:
2988:
2984:
2980:
2976:
2972:
2968:
2964:
2960:
2956:
2952:
2948:
2944:
2940:
2935:
2930:
2929:
2927:
2921:
2917:
2913:
2908:
2904:
2900:
2895:
2894:
2891:
2888:
2876:
2875:
2874:
2873:
2868:
2864:
2853:
2852:
2851:
2846:
2842:
2838:
2830:
2826:
2822:
2818:
2812:
2808:
2804:
2800:
2784:
2781:
2772:
2764:
2756:
2752:
2740:
2730:
2727:
2726:
2724:
2719:
2715:
2711:
2707:
2703:
2698:
2693:
2692:
2690:
2685:
2681:
2677:
2673:
2669:
2664:
2659:
2658:
2656:
2651:
2650:+ 2 Cs[AsF
2647:
2643:
2642:] + Ni[AsF
2639:
2635:
2631:
2627:
2623:
2619:
2612:
2607:
2602:
2601:
2599:
2594:
2590:
2586:
2582:
2578:
2574:
2569:
2564:
2563:
2561:
2556:
2552:
2548:
2544:
2540:
2535:
2530:
2529:
2527:
2522:
2518:
2514:
2510:
2506:
2501:
2496:
2495:
2493:
2488:
2484:
2480:
2476:
2472:
2467:
2462:
2461:
2459:
2454:
2450:
2446:
2442:
2438:
2433:
2428:
2427:
2425:
2420:
2416:
2412:
2408:
2404:
2399:
2394:
2393:
2388:
2383:
2378:
2374:
2370:
2366:
2361:
2356:
2355:
2352:
2347:
2343:
2339:
2335:
2330:
2325:
2324:
2322:
2317:
2313:
2309:
2305:
2301:
2296:
2291:
2290:
2288:
2283:
2279:
2275:
2271:
2267:
2263:
2259:
2254:
2249:
2248:
2246:
2241:
2237:
2233:
2229:
2225:
2220:
2215:
2214:
2212:
2208:
2207:] + 3 Xe + BrF
2204:
2200:
2196:
2192:
2188:
2183:
2178:
2177:
2173:
2168:
2164:
2151:
2147:
2143:
2139:
2135:
2130:
2125:
2124:
2121:
2116:
2112:
2108:
2104:
2100:
2095:
2090:
2089:
2087:
2082:
2078:
2074:
2070:
2066:
2062:
2058:
2053:
2048:
2047:
2045:
2041:
2037:
2033:
2029:
2025:
2021:
2017:
2012:
2007:
2006:
2003:
1998:
1994:
1990:
1986:
1982:
1977:
1972:
1971:
1965:
1959:
1954:
1950:
1946:
1942:
1938:
1934:
1930:
1925:
1920:
1919:
1916:
1915:(not balanced)
1910:
1906:
1902:
1898:
1894:
1890:
1886:
1881:
1876:
1875:
1872:
1867:
1863:
1859:
1855:
1851:
1847:
1843:
1837:
1833:
1825:
1820:
1819:
1816:
1813:
1804:
1800:
1799:
1794:
1790:
1786:
1782:
1778:
1773:
1769:
1765:
1761:
1757:
1753:
1749:
1744:
1740:
1736:
1732:
1722:
1718:
1714:
1706:
1702:
1698:
1693:
1692:
1688:
1684:
1680:
1675:
1669:
1657:
1656:
1651:
1647:
1643:
1639:
1634:
1633:
1629:
1628:
1620:
1613:
1607:
1601:
1596:
1595:
1555:
1552:
1551:
1550:
1545:
1541:
1537:
1533:
1528:
1524:
1520:
1516:
1511:
1507:
1503:
1499:
1491:
1484:
1478:
1457:
1454:
1439:
1431:
1423:
1407:
1381:
1366:
1358:
1342:
1339:is ionized to
1335:
1313:
1310:
1302:
1291:
1278:Main article:
1275:
1272:
1264:
1260:
1256:
1248:
1233:
1217:
1213:
1205:
1201:
1188:
1173:
1164:
1156:
1147:
1144:
1143:
1138:
1134:
1130:
1129:
1124:
1120:
1116:
1105:
1101:
1097:
1093:
1089:
1088:
1083:
1076:
1072:
1071:
1066:
1062:
1058:
1054:
1050:
1049:
1044:
1040:
1036:
1032:
1028:
1024:
1023:
1018:
1014:
1010:
1006:
1002:
998:
994:
990:
986:
982:
981:
976:
972:
968:
964:
960:
956:
952:
948:
944:
935:
928:
924:
920:
916:
912:
908:
904:
896:
892:
884:
880:
876:
868:
848:
840:
813:
789:
785:
777:
773:
757:
753:
749:
741:
737:
721:
713:
709:
705:
687:
684:
681:
680:
675:
671:
667:
666:
661:
657:
653:
649:
645:
641:
637:
636:
631:
627:
623:
622:
617:
613:
609:
605:
601:
597:
593:
589:
585:
581:
577:
573:
569:
565:
561:
557:
553:
549:
548:
543:
539:
535:
531:
527:
523:
519:
515:
511:
507:
503:
492:
491:
486:
482:
478:
474:
470:
466:
462:
458:
454:
450:
446:
442:
438:
437:Higher species
434:
433:
428:
424:
420:
416:
415:
410:
406:
402:
401:
396:
392:
388:
387:
382:
378:
374:
370:
369:
364:
360:
356:
352:
341:
340:
335:
331:
327:
323:
319:
318:
313:
309:
305:
301:
297:
293:
289:
288:
283:
279:
275:
271:
267:
263:
259:
255:
251:
234:
218:
210:
203:
191:
187:
179:
170:
167:
166:
161:
157:
156:Higher species
153:
152:
147:
140:
136:
135:
130:
126:
122:
118:
117:
112:
108:
104:
100:
99:
94:
90:
86:
82:
78:
77:
72:
68:
64:
57:
48:
43:
40:
9:
6:
4:
3:
2:
4787:
4776:
4773:
4772:
4770:
4756:
4749:
4747:
4745:
4743:
4741:
4732:
4728:
4724:
4720:
4716:
4712:
4708:
4704:
4697:
4686:
4678:
4674:
4670:
4666:
4662:
4658:
4654:
4650:
4646:
4642:
4638:
4631:
4623:
4621:0-12-352651-5
4617:
4613:
4606:
4604:
4595:
4589:
4585:
4578:
4576:
4574:
4572:
4570:
4568:
4566:
4564:
4555:
4549:
4545:
4541:
4537:
4531:
4529:
4527:
4525:
4523:
4521:
4519:
4510:
4504:
4500:
4493:
4491:
4489:
4487:
4485:
4483:
4481:
4479:
4477:
4468:
4466:9780470862100
4462:
4458:
4451:
4449:
4447:
4445:
4440:
4430:
4427:
4425:
4422:
4420:
4417:
4415:
4412:
4410:
4407:
4406:
4400:
4374:
4371:and the most
4366:
4362:
4358:
4351:
4345:
4339:
4306:
4305:
4280:
4279:
4278:
4276:
4241:
4188:
4164:
4163:
4162:
4160:
4156:
4145:
4134:
4111:F] → [ClF
4105:
4104:
4103:
4101:
4083:
4059:
4055:
4040:
4019:
4018:
4017:
3981:
3978:
3968:
3958:
3948:
3926:
3904:
3894:
3884:
3874:
3872:is dark brown
3864:
3854:
3852:is cherry red
3844:
3834:
3824:
3806:compounds of
3805:
3802:
3801:fluorocations
3799:
3798:
3797:
3795:
3783:
3773:
3751:] > [I
3737:
3736:
3735:
3733:
3712:
3711:
3710:
3701:
3697:
3688:
3656:
3652:
3640:
3637:is stable in
3628:
3615:
3581:
3576:
3574:
3561:
3545:
3543:
3508:
3504:
3475:
3466:
3465:
3462:
3443:
3439:
3426:
3425:
3422:
3409:
3400:
3399:
3396:
3363:
3354:
3353:
3350:
3337:
3328:
3327:
3323:
3294:
3285:
3284:
3273:
3259:→ 6 K[BrF
3255:6 KCl + 8 BrF
3253:
3244:
3243:
3240:
3227:
3218:
3217:
3214:
3209:
3205:
3192:
3191:
3188:
3175:
3166:
3165:
3162:
3149:
3140:
3139:
3136:
3119:
3110:
3109:
3106:
3097:
3088:
3087:
3077:
3024:
3015:
3014:
3002:
2998:
2994:
2941:
2932:
2931:
2928:
2914:
2897:
2896:
2892:
2889:
2886:
2885:
2879:
2861:
2860:
2858:
2854:
2839:
2833:
2815:
2797:
2796:
2794:
2790:
2789:
2788:
2778:
2776:
2748:
2725:
2704:
2695:
2694:
2691:
2670:
2661:
2660:
2657:
2616:
2613:
2604:
2603:
2600:
2575:
2566:
2565:
2562:
2541:
2532:
2531:
2528:
2507:
2498:
2497:
2494:
2473:
2464:
2463:
2460:
2439:
2430:
2429:
2426:
2405:
2396:
2395:
2384:
2367:
2358:
2357:
2353:
2336:
2327:
2326:
2323:
2302:
2293:
2292:
2289:
2260:
2251:
2250:
2247:
2226:
2217:
2216:
2213:
2199:] → 3 [Br
2189:
2180:
2179:
2169:
2136:
2127:
2126:
2122:
2101:
2092:
2091:
2088:
2059:
2050:
2049:
2046:
2032:] → 2 [Br
2018:
2009:
2008:
2004:
1983:
1974:
1973:
1970:
1960:
1931:
1922:
1921:
1917:
1887:
1878:
1877:
1873:
1844:
1822:
1821:
1817:
1814:
1811:
1810:
1803:
1779:
1750:
1729:
1728:
1727:
1678:
1666:
1665:
1664:
1662:
1636:
1635:
1631:
1630:
1623:
1616:
1610:
1604:
1598:
1597:
1593:
1589:
1585:
1581:
1577:
1573:
1569:
1565:
1561:
1560:
1559:
1534:
1517:
1500:
1494:
1487:
1481:
1475:
1474:
1473:
1471:
1467:
1466:interhalogens
1463:
1453:
1451:
1445:
1419:
1415:
1399:
1395:
1393:
1389:
1377:
1371:
1354:
1353:bond strength
1350:
1331:
1326:
1324:
1320:
1309:
1287:
1281:
1243:
1239:
1229:
1225:
1194:
1179:
1167:
1135:
1132:
1131:
1113:
1094:
1091:
1090:
1080:
1077:
1074:
1073:
1055:
1052:
1051:
1029:
1027:Square planar
1026:
1025:
1013:Cl], [IBr
987:
984:
983:
945:
942:
941:
934:
864:
860:
856:
836:
832:
827:
824:[Cl−F−Cl]
820:[Cl−Cl−F]
809:
805:
801:
768:
732:
700:
692:
672:
669:
668:
642:
639:
638:
628:
625:
624:
576:], [IBrCl
554:
551:
550:
504:
501:
500:
439:
436:
435:
421:
418:
417:
407:
404:
403:
393:
390:
389:
375:
372:
371:
353:
350:
349:
324:
321:
320:
294:
291:
290:
252:
249:
248:
240:
224:
199:
158:
155:
154:
144:
141:
138:
137:
123:
120:
119:
105:
102:
101:
83:
80:
79:
61:
58:
55:
54:
47:
39:
37:
33:
29:
25:
19:
4754:
4706:
4702:
4685:
4644:
4640:
4630:
4611:
4583:
4539:
4498:
4456:
4414:Halogen bond
4364:
4360:
4356:
4349:
4343:
4337:
4331:
4242:
4189:
4178:
4133:paramagnetic
4123:
4079:
4025:+ 2 [BrF
3990:
3779:
3771:
3729:
3650:
3616:
3577:
3556:
3513:
3507:acetonitrile
3369:+ 2 [(CH
3181:→ Cs[IBr
2920:+ X → [X
2877:
2845:+ X → [X
2831:
2813:
2786:
2746:
2731:
2483:F → [IBr
2385:with excess
2232:+ ICl + AlCl
1801:
1789:] → [BrF
1768:] + [ClF
1739:F → [IBr
1694:
1658:
1621:
1614:
1608:
1602:
1564:interhalogen
1557:
1544:] + 2 [X
1492:
1485:
1479:
1459:
1446:
1403:
1372:
1327:
1315:
1283:
1227:
1223:
1222:ions have a
1197:
1180:
1165:
1148:
997:F], [BrF
955:], [BrCl
862:
828:
797:
572:F], [ICl
514:], [BrCl
262:F], [BrF
225:
171:
45:
42:Introduction
23:
22:
4232:instead of
4144:diamagnetic
4115:] + [Cl
3980:polyiodides
3544:is cooled.
3343:→ K[ICl
3155:→ K[ICl
2867:→ K[ICl
2338:2 ClF + AsF
1989:+ ClF + AsF
1858:] → [Cl
1705:], [BrF
1701:], [ClF
1642:+ ClF + AsF
1619:] + [MY
1490:] + [XY
1418:wavenumbers
1119:], [BrF
1100:], [BrF
1061:], [BrF
1043:], [ICl
1035:], [BrF
1005:], [ICl
975:Cl], [I
967:], [IBr
963:], [ICl
951:], [BrF
919:], [BrF
911:], [ClF
831:VSEPR model
712:], [ICl
708:], [ClF
648:], [BrF
616:Cl], [I
604:Cl], [I
568:], [ICl
560:], [BrF
534:Cl], [I
522:], [ICl
510:], [BrF
330:], [BrF
300:], [BrF
282:Cl], [I
270:], [ICl
34:containing
4435:References
4424:Catenation
4409:Polyiodide
4385:N][ClF
4170:] ⇌ [I
3918:][AlCl
3548:Properties
3481:+ [(CH
3300:+ [(CH
3030:+ [(CH
2999:or liquid
2947:+ [(CH
2903:], [Br
2817:→ [(CH
2793:Lewis base
2680:→ [BrF
2634:→ [ClF
2551:→ [BrF
2517:→ [ClF
2453:][SbCl
2449:→ [ICl
2346:F][AsF
2312:→ [ClF
2240:][AlCl
2170:in liquid
2077:→ 2 [I
2024:+ 2 [O
1949:→ 2 [I
1897:F) + 3 SbF
1760:→ [ClF
1679:→ 2 [I
1568:Lewis acid
1540:⇌ 2 [X
1527:] + [X
1510:] + [X
1450:difluorine
1349:bond order
1286:polyiodide
1280:Polyiodide
1123:], [IF
1104:], [IF
1092:Octahedral
1065:], [IF
1039:], [IF
1001:], [IF
993:], [Cl
959:], [IF
837:, such as
835:lone pairs
756:][AsCl
652:], [IF
564:], [IF
518:], [IF
359:], [Br
334:], [IF
304:], [IF
266:], [IF
258:], [Cl
89:], [Br
67:], [Br
18:Polyhalite
4677:155093006
4661:1433-7851
4429:Allotropy
4312:][AsF
4286:][PtF
4203:][IrF
4107:2 [Cl
4064:][SbF
4060:+ [IF
4045:] + 2 BrF
4029:][AsF
3832:is yellow
3732:symmetric
3714:14 [I
3627:superacid
3553:Stability
3497:N][IF
3316:N][IF
3229:NOF + ClF
3194:[AtBr
3112:[BrCl
3070:N][Br
2987:N][Br
2907:], [I
2829:N][XY
2718:][BrF
2714:→ [IF
2684:][AsF
2638:][AsF
2626:] + 5 AsF
2585:→ [IF
2555:][AsF
2521:][SbF
2419:][AsF
2415:→ [IF
2392:required
2377:][AuF
2342:→ [Cl
2316:][AsF
2203:][AsF
2150:][AsF
2115:][IrF
2111:→ [Cl
2036:][AsF
2028:][AsF
1997:][AsF
1993:→ [Cl
1901:→ [Br
1866:][SbF
1854:][SbF
1793:][AsF
1772:][PtF
1764:][PtF
1683:][SbF
1650:][AsF
1646:→ [Cl
1612:→ [XY
1483:⇌ [XY
1456:Synthesis
1017:], [I
1009:], [I
971:], [I
915:][SbF
907:][SbF
883:N][IF
788:N][IF
718:in their
686:Structure
656:], [I
612:], [I
596:], [I
588:], [I
580:], [I
530:], [I
485:], [I
481:], [I
477:], [I
473:], [I
469:], [I
465:], [I
461:], [I
457:], [I
453:], [I
449:], [I
445:], [I
427:], [I
381:], [I
363:], [I
308:], [I
278:], [I
129:], [I
111:], [I
93:], [I
71:], [I
4775:Halogens
4769:Category
4731:23159174
4723:15535716
4669:31090163
4403:See also
4393:[ClF
4377:[(CH
4355:, where
4308:[BrF
4282:[ClF
4267:[BrF
4259:[ClF
4166:2 [I
4041:[AsF
4002:[BrF
3994:[ClF
3970:[ICl
3960:[ICl
3956:is black
3940:][SO
3862:is brown
3808:[ICl
3565:[SbF
3451:P][I
3339:KI + ICl
3330:[ICl
3320:] + 2 Xe
3263:] + 3 Cl
3246:[BrF
3220:[ClF
3177:CsI + Br
3168:[IBr
3142:[ICl
3090:[ClF
2811:N]Y + XY
2799:[(CH
2755:[NiF
2739:[NiF
2663:[BrF
2622:[NiF
2606:[ClF
2589:][Sb
2534:[BrF
2500:[ClF
2487:][SO
2466:[IBr
2432:[ICl
2360:[BrF
2295:[ClF
2236:→ [I
2146:→ [I
2081:][SO
1953:][SO
1905:][Sb
1893:(in BrSO
1850:+ [O
1743:][SO
1697:[IBr
1661:fluoride
1523:⇌ [X
1506:⇌ [X
1470:halogens
1430:[ICl
1422:[ICl
1406:[ICl
1380:[ICl
1259:][Sb
1255:[BrF
1247:[BrF
1187:[ClF
1172:[XeF
1115:[ClF
1096:[ClF
1057:[ClF
1031:[ClF
989:[ClF
947:[ClF
923:][Sb
903:[BrF
875:[(CH
839:[BrF
822:but not
720:[SbF
704:[BrF
644:[ClF
556:[ClF
506:[ClF
326:[ClF
296:[ClF
254:[ClF
36:halogens
4335:M[X
4294:] + ClF
4234:[Cl
4226:[Cl
4199:[Cl
4191:[Cl
4086:[Cl
4010:[IF
3866:[Br
3856:[Br
3846:[Br
3842:is blue
3836:[Cl
3826:[Cl
3794:amylose
3608:[IF
3592:[XF
3527:K[I
3468:[IF
3402:[IF
3389:[IF
3356:[IF
3287:[IF
3281:needed
3274:excess
3151:KI + Cl
3017:[Br
3005:[Br
2934:[Br
2899:[Cl
2887:Species
2863:KI + Cl
2857:halides
2749:in the
2747:in situ
2697:[IF
2676:] + BrF
2581:+ 2 SbF
2568:[IF
2398:[IF
2329:[Cl
2182:[Br
2142:+ 3 AsF
2094:[Cl
2040:] + 2 O
2011:[Br
1976:[Cl
1880:[Br
1832:[Cl
1824:[Cl
1812:Species
1756:+ 2 PtF
1687:] + SbF
1566:with a
1438:[XY
1312:Bonding
1155:[IF
1137:[IF
1082:[IF
867:[IF
847:[IF
812:[Cl
784:[Me
674:[IF
630:[IF
423:[Br
377:[Br
355:[Cl
217:[Cl
215:. Free
209:[Cl
186:[Cl
178:[Cl
125:[Br
107:[Cl
85:[Cl
63:[Cl
28:cations
4729:
4721:
4675:
4667:
4659:
4618:
4590:
4550:
4505:
4463:
4273:using
4211:[I
4181:[I
4147:[I
4136:[I
4125:[I
4072:] + IF
4035:[O
4033:] → 2
3950:[I
3936:[I
3928:[I
3914:[I
3906:[I
3896:[I
3886:[I
3876:[I
3816:[I
3786:[I
3782:starch
3739:[I
3722:] + IF
3667:[I
3659:[I
3631:[I
3619:[X
3600:[X
3584:[X
3428:[I
2688:] + Kr
2479:+ IOSO
2445:+ SbCl
2253:[I
2219:[I
2129:[I
2052:[I
1924:[I
1797:] + Kr
1735:+ IOSO
1357:[X
1347:, the
1341:[X
1301:[I
1232:[I
1212:[I
1200:[I
891:[I
772:[I
748:[I
736:[I
726:salts.
441:[I
409:[I
395:[I
233:[I
160:[I
146:[I
32:anions
4727:S2CID
4699:(PDF)
4673:S2CID
4324:] + F
3776:Color
3580:oleum
3296:2 XeF
2710:+ BrF
2630:+ ClF
2547:+ AsF
2513:+ SbF
2411:+ AsF
2369:5 BrF
2308:+ AsF
2278:→ 2 I
2163:+ AsF
2107:+ IrF
1752:2 ClF
1721:, BrF
1717:, ClF
1332:when
1224:trans
200:from
4719:PMID
4665:PMID
4657:ISSN
4616:ISBN
4588:ISBN
4548:ISBN
4503:ISBN
4461:ISBN
4265:and
4250:and
4157:and
4008:and
3673:and
3606:and
3590:and
3563:and
3540:and
3518:and
3267:+ Br
3125:+ Cl
3026:2 Br
2191:8 Br
2103:2 Cl
2020:3 Br
1830:(as
1672:+ 3
1606:+ MY
1477:2 XY
1299:and
1284:The
1210:and
1198:The
985:Bent
845:and
806:and
592:BrCl
30:and
4711:doi
4707:126
4649:doi
4298:+ F
4275:NOF
4252:BrF
4245:ClF
4197:in
4096:SbF
4094:HF/
4049:+ F
4021:2 O
3820:Cl]
3767:Cl]
3704:SbF
3700:NaF
3698:or
3692:TaF
3683:NbF
3643:SbF
3505:in
3276:BrF
2995:in
2771:PtF
2763:AsF
2543:BrF
2509:ClF
2441:ICl
2387:BrF
2304:ClF
2266:+ S
2262:7 I
2228:2 I
2138:2 I
2065:+ S
2061:3 I
1964:HSO
1961:in
1937:+ S
1933:2 I
1781:BrF
1713:IBr
1674:SbF
1668:3 I
1536:5 X
1519:4 X
1502:3 X
1468:or
1414:ICl
1355:in
1253:in
979:Br]
863:via
746:in
620:Br]
286:Br]
207:to
4771::
4739:^
4725:.
4717:.
4705:.
4701:.
4671:.
4663:.
4655:.
4645:59
4643:.
4639:.
4602:^
4562:^
4517:^
4475:^
4443:^
4363:+
4359:+
4240:.
4219:Cl
4187:.
4090:F]
4058:Rn
4000:,
3952:15
3944:F]
3709:.
3689:,
3665:,
3648:,
3614:.
3560:Cs
3542:KI
3477:IF
3455:Br
3442:Ph
3432:Br
3411:IF
3385:N]
3365:IF
3208:At
3121:Br
3080:Br
3062:CH
3058:CH
3054:CH
3042:CH
3038:CH
3034:CH
3003:.
2979:CH
2975:CH
2971:CH
2959:CH
2955:CH
2951:CH
2943:Br
2859::
2834:+1
2821:CH
2803:CH
2777:.
2751:Cs
2706:IF
2618:Cs
2595:11
2577:IF
2491:F]
2475:Br
2407:IF
2333:F]
2282:SO
2172:SO
2085:F]
1985:Cl
1957:F]
1911:16
1889:Br
1846:Cl
1747:F]
1731:Br
1638:Cl
1624:+1
1617:−1
1600:XY
1592:HF
1588:Sb
1586:,
1584:As
1582:,
1578:,
1576:Al
1574:,
1495:+1
1488:−1
1472::
1394::
1370:.
1295:,
1265:11
1238:.
1216:Br
1204:Cl
1178:.
1163:XY
1110:,
933:.
929:11
897:11
826:.
816:F]
802:,
778:12
752:Cl
740:Cl
660:Br
608:Br
600:Br
584:Cl
487:29
483:28
479:26
475:26
471:22
467:16
463:13
459:12
455:11
451:10
447:10
312:Cl
162:15
4733:.
4713::
4694:3
4679:.
4651::
4624:.
4596:.
4556:.
4511:.
4469:.
4397:]
4395:6
4389:]
4387:4
4383:4
4381:)
4379:3
4369:M
4365:p
4361:n
4357:m
4353:]
4350:p
4347:Z
4344:n
4341:Y
4338:m
4326:2
4322:6
4318:6
4314:6
4310:6
4300:2
4296:5
4292:6
4288:6
4284:6
4271:]
4269:6
4263:]
4261:6
4254:7
4247:7
4238:]
4236:2
4230:]
4228:3
4221:2
4215:]
4213:4
4207:]
4205:6
4201:4
4195:]
4193:4
4185:]
4183:2
4174:]
4172:4
4168:2
4151:]
4149:2
4140:]
4138:2
4129:]
4127:2
4119:]
4117:3
4113:2
4109:2
4098:5
4088:2
4074:5
4070:6
4066:6
4062:6
4051:2
4047:5
4043:6
4039:]
4037:2
4031:6
4027:6
4023:2
4014:]
4012:6
4006:]
4004:6
3998:]
3996:6
3974:]
3972:4
3964:]
3962:2
3954:]
3942:3
3938:7
3932:]
3930:7
3922:]
3920:4
3916:5
3910:]
3908:5
3900:]
3898:4
3890:]
3888:3
3880:]
3878:2
3870:]
3868:5
3860:]
3858:3
3850:]
3848:2
3840:]
3838:4
3830:]
3828:3
3818:2
3812:]
3810:2
3790:]
3788:5
3765:2
3761:2
3757:3
3753:2
3749:2
3745:2
3741:3
3724:5
3720:3
3716:2
3706:5
3694:5
3685:5
3677:2
3675:I
3671:]
3669:5
3663:]
3661:3
3654:0
3651:H
3645:5
3635:]
3633:2
3623:]
3621:2
3612:]
3610:6
3604:]
3602:3
3596:]
3594:6
3588:]
3586:2
3569:]
3567:6
3537:2
3535:I
3531:]
3529:3
3522:2
3520:I
3516:I
3501:]
3499:8
3495:4
3493:)
3491:3
3487:4
3485:)
3483:3
3479:7
3472:]
3470:8
3459:]
3457:4
3453:3
3449:4
3445:4
3436:]
3434:4
3430:3
3419:]
3417:6
3413:5
3406:]
3404:6
3393:]
3391:5
3387:2
3383:4
3381:)
3379:3
3375:4
3373:)
3371:3
3367:3
3360:]
3358:5
3347:]
3345:4
3341:3
3334:]
3332:4
3318:4
3314:4
3312:)
3310:3
3306:4
3304:)
3302:3
3298:2
3291:]
3289:4
3278:3
3269:2
3265:2
3261:4
3257:3
3250:]
3248:4
3237:]
3235:4
3231:3
3224:]
3222:4
3202:]
3200:2
3196:2
3185:]
3183:2
3179:2
3172:]
3170:2
3159:]
3157:2
3153:2
3146:]
3144:2
3133:]
3131:2
3127:2
3123:2
3116:]
3114:2
3103:]
3101:2
3094:]
3092:2
3082:2
3074:]
3072:5
3068:4
3066:)
3064:2
3060:2
3056:2
3052:3
3048:4
3046:)
3044:2
3040:2
3036:2
3032:3
3028:2
3021:]
3019:5
3009:]
3007:3
2991:]
2989:3
2985:4
2983:)
2981:2
2977:2
2973:2
2969:3
2965:4
2963:)
2961:2
2957:2
2953:2
2949:3
2945:2
2938:]
2936:3
2924:]
2922:3
2918:2
2916:X
2911:]
2909:3
2905:3
2901:3
2871:]
2869:2
2865:2
2849:]
2847:3
2843:2
2841:X
2836:]
2832:n
2827:4
2825:)
2823:2
2819:3
2814:n
2809:4
2807:)
2805:2
2801:3
2773:6
2765:5
2761:/
2759:]
2757:6
2753:2
2743:]
2741:3
2734:‡
2722:]
2720:4
2716:6
2712:3
2708:7
2701:]
2699:6
2686:6
2682:6
2678:5
2674:6
2667:]
2665:6
2654:]
2652:6
2648:2
2646:]
2644:6
2640:6
2636:6
2632:5
2628:5
2624:6
2620:2
2615:‡
2610:]
2608:6
2597:]
2593:F
2591:2
2587:4
2583:5
2579:5
2572:]
2570:4
2559:]
2557:6
2553:4
2549:5
2545:5
2538:]
2536:4
2525:]
2523:6
2519:4
2515:5
2511:5
2504:]
2502:4
2489:3
2485:2
2481:2
2477:2
2470:]
2468:2
2457:]
2455:6
2451:2
2447:5
2443:3
2436:]
2434:2
2423:]
2421:6
2417:2
2413:5
2409:3
2402:]
2400:2
2389:3
2381:]
2379:4
2375:2
2371:3
2364:]
2362:2
2350:]
2348:6
2344:2
2340:5
2331:2
2320:]
2318:6
2314:2
2310:5
2306:3
2299:]
2297:2
2286:F
2284:3
2280:7
2276:2
2274:F
2272:6
2270:O
2268:2
2264:2
2257:]
2255:7
2244:]
2242:4
2238:5
2234:3
2230:2
2223:]
2221:5
2209:3
2205:6
2201:5
2197:6
2193:2
2186:]
2184:5
2174:2
2165:3
2160:2
2157:−
2154:]
2152:6
2148:4
2144:5
2140:2
2133:]
2131:4
2119:]
2117:6
2113:4
2109:6
2105:2
2098:]
2096:4
2083:3
2079:3
2075:2
2073:F
2071:6
2069:O
2067:2
2063:2
2056:]
2054:3
2042:2
2038:6
2034:3
2030:6
2026:2
2022:2
2015:]
2013:3
2001:]
1999:6
1995:3
1991:5
1987:2
1980:]
1978:3
1968:F
1966:3
1955:3
1951:2
1947:2
1945:F
1943:6
1941:O
1939:2
1935:2
1928:]
1926:2
1913:]
1909:F
1907:3
1903:2
1899:5
1895:3
1891:2
1884:]
1882:2
1870:]
1868:6
1864:2
1862:O
1860:2
1856:6
1852:2
1848:2
1842:)
1840:]
1838:2
1836:O
1834:2
1828:]
1826:2
1795:6
1791:6
1787:6
1783:5
1776:]
1774:6
1770:4
1766:6
1762:6
1758:6
1754:5
1745:3
1741:2
1737:2
1733:2
1723:7
1719:7
1715:3
1709:]
1707:6
1703:6
1699:2
1689:3
1685:6
1681:3
1676:5
1670:2
1654:]
1652:6
1648:3
1644:5
1640:2
1626:]
1622:m
1615:n
1609:m
1603:n
1580:P
1572:B
1548:]
1546:3
1542:2
1538:2
1531:]
1529:3
1525:5
1521:2
1514:]
1512:3
1508:3
1504:2
1497:]
1493:n
1486:n
1480:n
1442:]
1440:2
1434:]
1432:2
1426:]
1424:2
1410:]
1408:2
1384:]
1382:2
1367:2
1365:X
1361:]
1359:2
1345:]
1343:2
1336:2
1334:X
1305:]
1303:3
1297:I
1292:2
1290:I
1269:.
1267:]
1263:F
1261:2
1257:4
1251:]
1249:4
1236:]
1234:5
1228:Z
1226:-
1220:]
1218:2
1214:3
1208:]
1206:2
1202:3
1191:]
1189:6
1183:¶
1176:]
1174:5
1166:n
1159:]
1157:5
1151:‡
1141:]
1139:8
1127:]
1125:6
1121:6
1117:6
1112:¶
1108:]
1106:6
1102:6
1098:6
1086:]
1084:5
1079:‡
1069:]
1067:4
1063:4
1059:4
1047:]
1045:4
1041:4
1037:4
1033:4
1019:2
1015:2
1011:2
1007:2
1003:2
999:2
995:2
991:2
977:2
973:2
969:2
965:2
961:2
957:2
953:2
949:2
931:]
927:F
925:6
921:4
917:6
913:2
909:6
905:2
899:]
895:F
893:2
887:]
885:6
881:4
879:)
877:3
871:]
869:6
851:]
849:6
843:]
841:6
814:2
794:.
792:]
790:6
786:4
780:]
776:F
774:2
762:.
760:]
758:6
754:2
750:3
744:]
742:2
738:3
724:]
722:6
716:]
714:2
710:2
706:2
678:]
676:8
664:]
662:4
658:3
654:6
650:6
646:6
634:]
632:5
618:4
614:4
610:3
606:2
602:2
598:2
594:2
590:2
586:3
582:2
578:3
574:4
570:3
566:4
562:4
558:4
546:]
544:2
540:2
536:2
532:2
528:2
524:2
520:2
516:2
512:2
508:2
489:]
443:9
431:]
429:8
425:8
413:]
411:7
399:]
397:5
385:]
383:4
379:4
367:]
365:3
361:3
357:3
338:]
336:6
332:6
328:6
316:]
314:2
310:3
306:4
302:4
298:4
284:2
280:2
276:2
272:2
268:2
264:2
260:2
256:2
237:]
235:7
228:†
221:]
219:2
213:]
211:2
204:2
202:O
194:]
192:2
190:O
188:2
182:]
180:2
174:*
164:]
150:]
148:7
143:†
133:]
131:5
127:5
115:]
113:4
109:4
97:]
95:3
91:3
87:3
75:]
73:2
69:2
65:2
60:*
20:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.