996:
1004:
1433:. Residue 16, serine, has been shown to bind MgATP. Site-specific mutagenesis was used to demonstrate this fact. This has led to a model in which the serine remains coordinated to the Mg ion after phosphate hydrolysis in order to facilitate its association with a different phosphate of the now ADP molecule. MgATP binding also induces significant conformational changes within the Fe protein. Site-directed mutagenesis was employed to create mutants in which MgATP binds but does not induce a conformational change. Comparing
1017:
972:) type. Both form an assembly of two α subunits, two β subunits, and two δ (sometimes γ) subunits. The delta subunits are homologous to each other, and the alpha and beta subunits themselves are homologous to the ones found in MoFe nitrogenase. The gene clusters are also homologous, and these subunits are interchangeable to some degree. All nitrogenases use a similar Fe-S core cluster, and the variations come in the cofactor metal.
1225:
1335:
4864:
832:
1347:
metal is hydrogenated. In the alternating pathway, one hydrogen is added to the terminal nitrogen, then one hydrogen is added to the nitrogen directly bound to the metal. This alternating pattern continues until ammonia is released. Because each pathway favors a unique set of intermediates, attempts to determine which path is correct have generally focused on the isolation of said intermediates, such as the
4244:
29:
1052:, is the nitrogenase that has been studied the most extensively and thus is the most well characterized. Vanadium nitrogenase and iron-only nitrogenase can both be found in select species of Azotobacter as an alternative nitrogenase. Equations 1 and 2 show the balanced reactions of nitrogen fixation in molybdenum nitrogenase and vanadium nitrogenase respectively.
1130:(FeMo-co). Mo is replaced by V or Fe in vanadium nitrogenase and iron-only nitrogenase respectively. During catalysis, 2 equivalents of MgATP are hydrolysed which helps to decrease the potential of the to the Fe-S cluster and drive reduction of the P-cluster, and finally to the FeMo-co, where reduction of N
1917:
cells, ARA has been applied to a wide range of test systems, including field studies where other techniques are difficult to deploy. For example, ARA was used successfully to demonstrate that bacteria associated with rice roots undergo seasonal and diurnal rhythms in nitrogenase activity, which were
1367:
Specific support for the distal pathway has mainly stemmed from the work of
Schrock and Chatt, who successfully isolated the nitrido complex using Mo as the metal center in a model complex. Specific support for the alternating pathway stems from a few studies. Iron only model clusters have been shown
1933:
but not acetylene for nitrogenase (leading to overestimates of nitrogenase by ARA). Bottle or chamber-based assays may produce negative impacts on microbial systems as a result of containment or disruption of the microenvironment through handling, leading to underestimation of nitrogenase. Despite
1910:
is involved in other reactions in the cell, it is often desirable to label the substrate with N to provide accounting or "mass balance" of the added substrate. A more common assay, the acetylene reduction assay or ARA, estimates the activity of nitrogenase by taking advantage of the ability of the
1272:
or proceed with nitrogen binding and finish the catalytic cycle. This intermediate is proposed to contain the FeMo-co in its resting oxidation state with two bridging hydrides and two sulfur bonded protons. This intermediate was first observed using freeze quench techniques with a mutated protein in
1376:
clusters have also been shown to follow an alternating pathway for nitrogen fixation. The vanadium nitrogenase releases hydrazine, an intermediate specific to the alternating mechanism. However, the lack of characterized intermediates in the native enzyme itself means that neither pathway has been
1346:
complex is generally agreed upon, there are currently two hypotheses for the exact pathway in the second half of the mechanism: the "distal" and the "alternating" pathway. In the distal pathway, the terminal nitrogen is hydrogenated first, releases ammonia, then the nitrogen directly bound to the
1186:
Spectroscopic characterization of these intermediates has allowed for greater understanding of nitrogen reduction by nitrogenase, however, the mechanism remains an active area of research and debate. Briefly listed below are spectroscopic experiments for the intermediates before the addition of
1321:
The above intermediates suggest that the metal cluster is cycled between its original oxidation state and a singly reduced state with additional electrons being stored in hydrides. It has alternatively been proposed that each step involves the formation of a hydride and that the metal cluster
1125:
All nitrogenases are two-component systems made up of
Component I (also known as dinitrogenase) and Component II (also known as dinitrogenase reductase). Component I is a MoFe protein in molybdenum nitrogenase, a VFe protein in vanadium nitrogenase, and an Fe protein in iron-only nitrogenase.
1154:
kinetics measurements carried out in the 70's and 80's by Lowe, Thorneley, and others provided a kinetic basis for this process. The Lowe-Thorneley (LT) kinetic model was developed from these experiments and documents the eight correlated proton and electron transfers required throughout the
1404:
residues of the Fe protein are well understood by comparing to similar enzymes, while the interactions with the rest of the molecule are more elusive due to the lack of a Fe protein crystal structure with MgATP bound (as of 1996). Three protein residues have been shown to have significant
1452:
Nitrogenase is able to reduce acetylene, but is inhibited by carbon monoxide, which binds to the enzyme and thereby prevents binding of dinitrogen. Dinitrogen prevent acetylene binding, but acetylene does not inhibit binding of dinitrogen and requires only one electron for reduction to
1387:
1359:
in the alternating pathway. Attempts to isolate the intermediates in nitrogenase itself have so far been unsuccessful, but the use of model complexes has allowed for the isolation of intermediates that support both sides depending on the metal center used. Studies with
1285:
show that the hydrides bridge between two iron centers. Cryoannealing of the trapped intermediate at -20 °C results in the successive loss of two hydrogen equivalents upon relaxation, proving that the isolated intermediate is consistent with the
709:
3768:
Physiological analysis of nodules from LbRNAi plants revealed the crucial contribution of leghemoglobins to establishing low free-oxygen concentrations but high energy status in nodules, conditions that are necessary for effective
2878:
Igarashi RY, Laryukhin M, Dos Santos PC, Lee HI, Dean DR, Seefeldt LC, Hoffman BM (May 2005). "Trapping H- bound to the nitrogenase FeMo-cofactor active site during H2 evolution: characterization by ENDOR spectroscopy".
848:
The Fe protein, the dinitrogenase reductase or NifH, is a dimer of identical subunits which contains one cluster and has a mass of approximately 60-64kDa. The function of the Fe protein is to transfer electrons from a
787:
1377:
definitively proven. Furthermore, computational studies have been found to support both sides, depending on whether the reaction site is assumed to be at Mo (distal) or at Fe (alternating) in the MoFe cofactor.
3738:
Ott, Thomas; van Dongen, Joost T.; Gu¨nther, Catrin; Krusell, Lene; Desbrosses, Guilhem; Vigeolas, Helene; Bock, Vivien; Czechowski, Tomasz; Geigenberger, Peter; Udvardi, Michael K. (March 29, 2005).
2789:
Yoo SJ, Angove HC, Papaefthymiou V, Burgess BK, Münck E (May 2000). "Mössbauer Study of the MoFe
Protein of Nitrogenase from Azotobacter vinelandii Using Selective 57Fe Enrichment of the M-Centers".
1911:
enzyme to reduce acetylene gas to ethylene gas. These gases are easily quantified using gas chromatography. Though first used in a laboratory setting to measure nitrogenase activity in extracts of
1429:, MgATP cannot bind due to the salt bridge being too strong. The necessity of specifically aspartic acid at site 125 has been shown through noting altered reactivity upon mutation of this residue to
2680:
Wilson PE, Nyborg AC, Watt GD (July 2001). "Duplication and extension of the
Thorneley and Lowe kinetic model for Klebsiella pneumoniae nitrogenase catalysis using a MATHEMATICA software platform".
909:, within the α subunits. The oxidation state of Mo in these nitrogenases was formerly thought Mo(V), but more recent evidence is for Mo(III). (Molybdenum in other enzymes is generally bound to
948:
Electrons from the Fe protein enter the MoFe protein at the P-clusters, which then transfer the electrons to the FeMo cofactors. Each FeMo cofactor then acts as a site for nitrogen fixation, with N
1171:
is also possible. Notably, nitrogen reduction has been shown to require 8 equivalents of protons and electrons as opposed to the 6 equivalents predicted by the balanced chemical reaction.
1146:
The reduction of nitrogen to two molecules of ammonia is carried out at the FeMo-co of
Component I after the sequential addition of proton and electron equivalents from Component II.
893:. The hydrolysis of ATP also causes a conformational change within the nitrogenase complex, bringing the Fe protein and MoFe protein closer together for easier electron transfer.
1437:
data in the mutants versus in the wild-type protein led to the conclusion that the entire protein contracts upon MgATP binding, with a decrease in radius of approximately 2.0 Å.
2916:"57Fe ENDOR spectroscopy and 'electron inventory' analysis of the nitrogenase E4 intermediate suggest the metal-ion core of FeMo-cofactor cycles through only one redox couple"
3414:
Georgiadis MM, Komiya H, Chakrabarti P, Woo D, Kornuc JJ, Rees DC (September 1992). "Crystallographic structure of the nitrogenase iron protein from
Azotobacter vinelandii".
936:
C) consists of two non-identical clusters: and , which are linked by three sulfide ions. Each FeMo cofactor is covalently linked to the α subunit of the protein by one
1925:
reduced (particularly in the case of ARA), is not always straightforward and may either underestimate or overestimate the true rate for a variety of reasons. For example, H
1400:
group of MgATP provides the energy needed to transfer electrons from the Fe protein to the MoFe protein. The binding interactions between the MgATP phosphate groups and the
901:
The MoFe protein is a heterotetramer consisting of two α subunits and two β subunits, with a mass of approximately 240-250kDa. The MoFe protein also contains two
3459:"Mapping the site(s) of MgATP and MgADP interaction with the nitrogenase of Azotobacter vinelandii. Lysine 15 of the iron protein plays a major role in MgATP interaction"
1386:
3916:
Rasche ME, Seefeldt LC (July 1997). "Reduction of thiocyanate, cyanate, and carbon disulfide by nitrogenase: kinetic characterization and EPR spectroscopic analysis".
1126:
Component II is a Fe protein that contains the Fe-S cluster., which transfers electrons to
Component I. Component I contains 2 metal clusters: the P-cluster, and the
495:
308:
175:
3876:
Seefeldt LC, Rasche ME, Ensign SA (April 1995). "Carbonyl sulfide and carbon dioxide as new substrates, and carbon disulfide as a new inhibitor, of nitrogenase".
1036:) and a process vital to sustaining life on Earth. There are three types of nitrogenase found in various nitrogen-fixing bacteria: molybdenum (Mo) nitrogenase,
626:
1007:
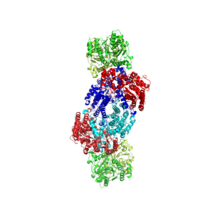
Nitrogenase with one set of metal clusters magnified. Electrons travel from the Fe-S cluster (yellow) to the P cluster (red), and end at the FeMo-co (orange).
1242:
and the additional proton bonded to a sulfur atom. Isolation of this intermediate in mutated enzymes shows that the FeMo-co is high spin and has a spin of /
2970:
Neese F (December 2005). "The
Yandulov/Schrock cycle and the nitrogenase reaction: pathways of nitrogen fixation studied by density functional theory".
1159:
where n = 0–8, corresponding to the number of equivalents transferred. The transfer of four equivalents are required before the productive addition of N
4523:
4496:
4447:
4386:
3542:
Wolle D, Dean DR, Howard JB (November 1992). "Nucleotide-iron-sulfur cluster signal transduction in the nitrogenase iron-protein: the role of Asp125".
2758:
Barney BM, Lee HI, Dos Santos PC, Hoffman BM, Dean DR, Seefeldt LC (May 2006). "Breaking the N2 triple bond: insights into the nitrogenase mechanism".
925:) of the P-cluster takes the form of two cubes linked by a central sulfur atom. Each P-cluster is linked to the MoFe protein by six cysteine residues.
4401:
3500:"Evidence for a central role of lysine 15 of Azotobacter vinelandii nitrogenase iron protein in nucleotide binding and protein conformational changes"
4528:
4424:
2818:"Connecting nitrogenase intermediates with the kinetic scheme for N2 reduction by a relaxation protocol and identification of the N2 binding state"
960:
The MoFe protein can be replaced by alternative nitrogenases in environments low in the Mo cofactor. Two types of such nitrogenases are known: the
718:
4276:
1898:
As with many assays for enzyme activity, it is possible to estimate nitrogenase activity by measuring the rate of conversion of the substrate (N
4299:
1471:. Despite this problem, many use oxygen as a terminal electron acceptor for respiration. Although the ability of some nitrogen fixers such as
1238:– This intermediate is proposed to contain the metal cluster in its resting oxidation state with the two added electrons stored in a bridging
4040:"Expression and association of group IV nitrogenase NifD and NifH homologs in the non-nitrogen-fixing archaeon Methanocaldococcus jannaschii"
3699:"Respiratory protection of nitrogenase in Azotobacter species: is a widely held hypothesis unequivocally supported by experimental evidence?"
194:
2586:
Schmidt, Frederik V.; Schulz, Luca; Zarzycki, Jan; Prinz, Simone; Oehlmann, Niels N.; Erb, Tobias J.; Rebelein, Johannes G. (2023-12-07).
4367:
1281:
experiments have provided insight into the structure of this intermediate, revealing the presence of two bridging hydrides. Mo and Fe
2458:
Franche C, Lindström K, Elmerich C (December 2008). "Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants".
3740:"Symbiotic Leghemoglobins Are Crucial for Nitrogen Fixation in Legume Root Nodules but Not for General Plant Growth and Development"
1483:, the effectiveness of such a mechanism has been questioned at oxygen concentrations above 70 μM (ambient concentration is 230 μM O
1465:, which degradatively oxidizes the Fe-S cofactors. This requires mechanisms for nitrogen fixers to protect nitrogenase from oxygen
1406:
4339:
3836:
Schrauzer GN (August 1975). "Nonenzymatic simulation of nitrogenase reactions and the mechanism of biological nitrogen fixation".
3623:"MgATP-induced conformational changes in the iron protein from Azotobacter vinelandii, as studied by small-angle x-ray scattering"
2715:
Simpson FB, Burris RH (June 1984). "A nitrogen pressure of 50 atmospheres does not prevent evolution of hydrogen by nitrogenase".
4391:
187:
3988:"Chloroplast-encoded chlB is required for light-independent protochlorophyllide reductase activity in Chlamydomonas reinhardtii"
1853:
The three subunits of nitrogenase exhibit significant sequence similarity to three subunits of the light-independent version of
1273:
which residue 70, a valine amino acid, is replaced with isoleucine. This modification prevents substrate access to the FeMo-co.
2521:
2217:
2184:
385:
256:
154:
1487:), as well as during additional nutrient limitations. A molecule found in the nitrogen-fixing nodules of leguminous plants,
515:
328:
4540:
987:
to function. An engineered minimal 10-gene operon that incorporates these additional essential genes has been constructed.
4269:
1934:
these weaknesses, such assays are very useful in assessing relative rates or temporal patterns in nitrogenase activity.
862:
2200:
Schneider K, Mueller A (2004). "Iron-Only
Nitrogenase: Exceptional Catalytic, Structural and Spectroscopic Features".
4889:
4583:
1282:
1278:
1020:
Catalytic sites within nitrogenase. Atoms are colored by element. Top: Fe-S Cluster Middle: P Cluster Bottom: FeMo-co
1268:, this intermediate is positioned after exactly half of the electron proton transfers and can either decay back to E
4550:
2118:
Bjornsson R, Delgado-Jaime MU, Lima FA, Sippel D, Schlesier J, Weyhermüller T, Einsle O, Neese F, DeBeer S (2015).
999:
Nitrogenase with catalytic sites highlighted. There are two sets of catalytic sites within each nitrogenase enzyme.
579:). Nitrogenases are the only family of enzymes known to catalyze this reaction, which is a step in the process of
4357:
4132:
Dilworth MJ (October 1966). "Acetylene reduction by nitrogen-fixing preparations from
Clostridium pasteurianum".
1311:
1274:
1197:
886:
1364:
generally point towards a distal pathway, while studies with Fe generally point towards an alternating pathway.
889:. The transfer of electrons requires an input of chemical energy which comes from the binding and hydrolysis of
148:
4262:
1778:
There are two types of bacteria that synthesize nitrogenase and are required for nitrogen fixation. These are:
870:
130:
2044:"Mechanisms for Generating Low Potential Electrons across the Metabolic Diversity of Nitrogen-Fixing Bacteria"
503:
316:
4739:
1948:
1878:
1854:
608:
135:
2235:"Reconstruction and minimal gene requirements for the alternative iron-only nitrogenase in Escherichia coli"
1255:– This intermediate is proposed to be the singly reduced FeMo-co with one bridging hydride and one hydride.
995:
4248:
2508:. Nitrogen Fixation: Origins, Applications, and Research Progress. Springer Netherlands. pp. 281–307.
619:
Although the equilibrium formation of ammonia from molecular hydrogen and nitrogen has an overall negative
4899:
4545:
3662:
Seefeldt LC, Dance IG, Dean DR (February 2004). "Substrate interactions with nitrogenase: Fe versus Mo".
3305:"Hydrazine is a product of dinitrogen reduction by the vanadium-nitrogenase from Azotobacter chroococcum"
1767:
1753:
1434:
199:
398:
123:
4854:
2379:
Peters JW, Szilagyi RK (April 2006). "Exploring new frontiers of nitrogenase structure and mechanism".
1745:
499:
312:
861:
to the nitrogenase protein. Ferredoxin or flavodoxin can be reduced by one of six mechanisms: 1. by a
4840:
4827:
4814:
4801:
4788:
4775:
4762:
4724:
1418:
405:
269:
58:
1221:
spectroscopy of the trapped intermediate indicates that the FeMo-co is integer spin greater than 1.
1218:
410:
4734:
4688:
4631:
4505:
4437:
4381:
4290:
1913:
1449:
remain unknown. No crystallographic analysis has been reported on substrate bound to nitrogenase.
1147:
874:
541:
535:
151:
41:
75:
4894:
4636:
4405:
4038:
Staples CR, Lahiri S, Raymond J, Von Herbulis L, Mukhophadhyay B, Blankenship RE (October 2007).
1392:
Binding of MgATP is one of the central events to occur in the mechanism employed by nitrogenase.
1003:
902:
2202:
Catalysts for Nitrogen Fixation: Nitrogenases, Relevant Chemical Models and Commercial Processes
2169:
Catalysts for Nitrogen Fixation: Nitrogenases, Relevant Chemical Models and Commercial Processes
2091:
Lawson DM, Smith BE (2002). "Molybdenum nitrogenases: a crystallographic and mechanistic view".
1425:, the protein's affinity for MgATP is greatly reduced and when the lysine is substituted for an
3219:
Chatt J, Dilworth JR, Richards RL (1978). "Recent advances in chemistry of nitrogen-fixation".
1737:
1499:
at the active site of the nitrogenase, while concomitantly allowing for efficient respiration.
905:, known as P-clusters, located at the interface between the α and β subunits and two
890:
560:
3951:
Guth JH, Burris RH (October 1983). "Inhibition of nitrogenase-catalyzed NH3 formation by H2".
1475:
to employ an oxygen-labile nitrogenase under aerobic conditions has been attributed to a high
793:, reducing this energy barrier such that the reaction can take place at ambient temperatures.
4657:
4576:
4088:
2914:
Doan PE, Telser J, Barney BM, Igarashi RY, Dean DR, Seefeldt LC, Hoffman BM (November 2011).
1795:
1348:
604:
583:. Nitrogen fixation is required for all forms of life, with nitrogen being essential for the
3248:"Reduction of N2 by supported tungsten clusters gives a model of the process by nitrogenase"
4729:
4312:
3621:
Chen L, Gavini N, Tsuruta H, Eliezer D, Burgess BK, Doniach S, Hodgson KO (February 1994).
3551:
3423:
3365:
3259:
3175:
3115:
2829:
2724:
2435:
2418:
2246:
1759:
1037:
961:
882:
620:
482:
295:
111:
3354:"Nitrogenase and nitrogenase reductase associate and dissociate with each catalytic cycle"
3012:
Hinnemann B, Nørskov JK (2008). "Catalysis by Enzymes: The Biological Ammonia Synthesis".
8:
4693:
4535:
4510:
1858:
878:
704:{\displaystyle \Delta H^{0}=-45.2\ \mathrm {kJ} \,\mathrm {mol^{-1}} \;\mathrm {NH_{3}} }
87:
53:
3555:
3427:
3369:
3263:
3179:
3119:
2833:
2728:
2618:
2250:
2068:
1869:, algae, and photosynthetic bacteria but has been lost by angiosperms during evolution.
390:
46:
4884:
4626:
4515:
4254:
4167:
Sims GK, Dunigan EP (1984). "Diurnal and seasonal variations in nitrogenase activity (C
4064:
4039:
3715:
3698:
3329:
3304:
3280:
3247:
3196:
3163:
3136:
3103:
3076:
3051:
3029:
2940:
2915:
2852:
2817:
2563:
2538:
2483:
2353:
2328:
2269:
2234:
2144:
2119:
1984:
1840:
4012:
3987:
3810:
3785:
3639:
3603:
3586:
3475:
3388:
3353:
2693:
4223:
4219:
4188:
4149:
4145:
4114:
4069:
4017:
3968:
3933:
3893:
3853:
3815:
3759:
3720:
3679:
3644:
3567:
3521:
3480:
3439:
3393:
3334:
3285:
3201:
3141:
3081:
2987:
2945:
2896:
2857:
2771:
2740:
2697:
2659:
2605:
2587:
2568:
2517:
2475:
2440:
2396:
2358:
2274:
2213:
2180:
2149:
2100:
2073:
2024:
1988:
1943:
1277:
characterization of this isolated intermediate shows a new species with a spin of ½.
1025:
1016:
712:
580:
490:
303:
261:
142:
3033:
2487:
2043:
444:
237:
4672:
4667:
4641:
4569:
4376:
4215:
4184:
4141:
4104:
4059:
4051:
4007:
3999:
3960:
3925:
3885:
3845:
3805:
3797:
3751:
3710:
3671:
3634:
3598:
3559:
3511:
3470:
3431:
3383:
3373:
3324:
3316:
3275:
3267:
3228:
3191:
3183:
3131:
3123:
3071:
3063:
3021:
2979:
2935:
2927:
2888:
2847:
2837:
2798:
2763:
2732:
2689:
2651:
2613:
2595:
2558:
2550:
2509:
2467:
2430:
2388:
2348:
2340:
2264:
2254:
2205:
2172:
2139:
2131:
2063:
2055:
2016:
2007:
1976:
1749:
1722:
1685:
1677:
1472:
816:
478:
291:
3801:
1921:
Unfortunately, the conversion of data from nitrogenase assays to actual moles of N
1322:
actually cycles between the original oxidation state and a singly oxidized state.
4719:
4703:
4616:
2513:
2209:
2176:
1741:
1681:
1338:
Distal vs. alternating mechanistic pathways for nitrogen fixation in nitrogenase.
1228:
Lowe-Thorneley kinetic model for reduction of nitrogen to ammonia by nitrogenase.
456:
366:
249:
4206:
Zumft WG, Mortenson LE (March 1975). "The nitrogen-fixing complex of bacteria".
3104:"N₂reduction and hydrogenation to ammonia by a molecular iron-potassium complex"
1213:– The one electron reduced intermediate has been trapped during turnover under N
1040:, and iron-only (Fe) nitrogenase. Molybdenum nitrogenase, which can be found in
4868:
4757:
4698:
4286:
3358:
Proceedings of the National Academy of Sciences of the United States of America
2822:
Proceedings of the National Academy of Sciences of the United States of America
2600:
2504:
Schneider K, Müller A (January 2004). Smith BE, Richards RL, Newton WE (eds.).
2392:
2239:
Proceedings of the National Academy of Sciences of the United States of America
1872:
Separately, two of the nitrogenase subunits (NifD and NifH) have homologues in
1694:
1476:
1224:
850:
394:
170:
3755:
3025:
2471:
4878:
4662:
4621:
4352:
3763:
3516:
3499:
2609:
2479:
1890:
they are known to interact with each other and are constitutively expressed.
1789:
1539:
1516:
1488:
1480:
1430:
1414:
910:
906:
812:
which has a high reducing power and is responsible for a supply of electrons.
556:
552:
373:
4109:
4092:
3739:
3563:
3435:
3127:
3052:"Catalytic reduction of dinitrogen to ammonia at a single molybdenum center"
2842:
2736:
2642:
Burgess BK, Lowe DJ (November 1996). "Mechanism of Molybdenum Nitrogenase".
2259:
782:{\displaystyle E_{\mathrm {A} }=230-420\ \mathrm {kJ} \,\mathrm {mol^{-1}} }
4611:
4464:
4118:
4073:
3849:
3724:
3683:
3378:
3289:
3205:
3145:
3085:
2991:
2983:
2949:
2900:
2861:
2775:
2701:
2663:
2572:
2444:
2400:
2362:
2278:
2153:
2135:
2104:
2077:
2028:
1824:
1151:
599:) that create plants, animals and other organisms. They are encoded by the
584:
4153:
4021:
4003:
3972:
3937:
3897:
3819:
3648:
3571:
3525:
3484:
3443:
3338:
2744:
2554:
2539:"The structure of vanadium nitrogenase reveals an unusual bridging ligand"
839:
showing the sites of binding to nitrogenase (the amino acids cys and his).
265:
4835:
4770:
4606:
4227:
3857:
3458:
3397:
2816:
Lukoyanov D, Barney BM, Dean DR, Seefeldt LC, Hoffman BM (January 2007).
2059:
1862:
1801:
1704:
866:
819:
MoFe protein, a nitrogenase which uses the electrons provided to reduce N
801:
596:
592:
4055:
3964:
3889:
3622:
3498:
Ryle MJ, Lanzilotta WN, Mortenson LE, Watt GD, Seefeldt LC (June 1995).
3232:
3187:
1519:. A list of other reactions carried out by nitrogenases is shown below:
544:
538:
99:
4451:
4428:
4303:
1980:
1873:
1866:
1733:
1512:
1401:
1393:
1361:
1041:
858:
854:
3929:
3675:
3587:"Nuclear Magnetic Resonance Spectra of Adenosine Di- and Triphosphate"
3320:
3271:
3164:"Catalytic conversion of nitrogen to ammonia by an iron model complex"
3067:
2931:
2892:
2802:
2655:
2344:
2020:
4809:
4783:
2767:
2327:
Hoffman BM, Lukoyanov D, Yang ZY, Dean DR, Seefeldt LC (April 2014).
1813:
1783:
1661:
1523:
1446:
1422:
1397:
1356:
941:
809:
588:
4863:
1527:
1462:
1454:
1426:
1373:
1334:
1196:– This is the resting state of the enzyme before catalysis begins.
1049:
984:
937:
790:
600:
564:
548:
451:
361:
244:
2005:
Burges BK, Lowe DJ (1996). "Mechanism of Molybdenum Nitrogenase".
1325:
378:
4455:
3102:
Rodriguez MM, Bill E, Brennessel WW, Holland PL (November 2011).
1835:
1818:
1763:
1714:
1650:
1579:
1566:
1508:
1467:
1352:
1239:
831:
805:
572:
531:
118:
4086:
4037:
2117:
827:. In some assemblies it is replaced by a homologous alternative.
214:
Nitrogenase-type Oxidoreductase (component 1 subunit alpha/beta)
4822:
4592:
4243:
2383:. Bioinorganic chemistry / Biocatalysis and biotransformation.
2329:"Mechanism of nitrogen fixation by nitrogenase: the next stage"
1590:
1458:
1410:
1127:
1045:
836:
510:
323:
182:
94:
82:
70:
4208:
Biochimica et Biophysica Acta (BBA) - Reviews on Bioenergetics
3413:
3101:
2877:
1882:. Little is understood about the function of these "class IV"
1507:
In addition to dinitrogen reduction, nitrogenases also reduce
1342:
While the mechanism for nitrogen fixation prior to the Janus E
28:
4796:
4484:
4479:
4474:
4343:
3786:"Interactions among substrates and inhibitors of nitrogenase"
2788:
2419:"Biosynthesis of the iron-molybdenum cofactor of nitrogenase"
1829:
1553:
1405:
interactions with the phosphates. In the absence of MgATP, a
1265:
3737:
3457:
Seefeldt LC, Morgan TV, Dean DR, Mortenson LE (April 1992).
4469:
4327:
4322:
4317:
3985:
3497:
3456:
2815:
1762:
have also been shown to catalyze the conversion of CO into
1492:
1421:
has demonstrated that when the lysine is substituted for a
472:
439:
285:
232:
106:
4561:
2757:
2585:
2233:
Yang J, Xie X, Wang X, Dixon R, Wang YP (September 2014).
4284:
3986:
Li J, Goldschmidt-Clermont M, Timko MP (December 1993).
3620:
2913:
2457:
2326:
1967:
Modak JM (2002). "Haber Process for Ammonia Synthesis".
1773:
1200:
characterization shows that this species has a spin of /
2588:"Structural insights into the iron nitrogenase complex"
4134:
Biochimica et Biophysica Acta (BBA) - General Subjects
1893:
4852:
3218:
1495:
prosthetic group, plays a crucial role in buffering O
721:
629:
3875:
3161:
1809:
Mutualistic bacteria (symbiotic), examples include:
1024:
Nitrogenase is an enzyme responsible for catalyzing
3162:Anderson JS, Rittle J, Peters JC (September 2013).
2120:"Molybdenum L-Edge XAS Spectra of MoFe Nitrogenase"
421:
Alternative nitrogenase (component 1) delta subunit
4387:4-Hydroxy-3-methylbut-2-enyl diphosphate reductase
3783:
3409:
3407:
1461:, most nitrogenases are irreversibly inhibited by
1155:reaction. Each intermediate stage is depicted as E
781:
703:
3661:
3245:
3097:
3095:
3007:
3005:
3003:
3001:
2499:
2497:
1886:genes, though they occur in many methanogens. In
1417:. Upon binding, this salt bridge is interrupted.
4876:
3157:
3155:
3011:
2873:
2871:
2679:
2232:
2199:
3541:
3404:
3045:
3043:
2637:
2635:
2633:
2631:
2629:
2503:
2322:
2320:
2318:
2316:
2314:
2312:
2310:
2308:
1848:
1141:
983:operon. This operon still requires some of the
952:binding in the central cavity of the cofactor.
614:
4205:
3915:
3092:
2998:
2675:
2673:
2494:
2378:
2374:
2372:
2306:
2304:
2302:
2300:
2298:
2296:
2294:
2292:
2290:
2288:
1174:
4577:
4270:
4033:
4031:
3537:
3535:
3351:
3152:
2965:
2963:
2961:
2959:
2868:
2714:
2412:
2410:
2041:
1380:
796:A usual assembly consists of two components:
3911:
3909:
3907:
3871:
3869:
3867:
3831:
3829:
3616:
3614:
3302:
3040:
2626:
2536:
2000:
1998:
1440:
4499:: Acting on X-H and Y-H to form an X-Y bond
4166:
2670:
2641:
2416:
2369:
2285:
2090:
2084:
343:Nitrogenase iron protein NifH (component 2)
4584:
4570:
4277:
4263:
4093:"The natural history of nitrogen fixation"
4028:
3950:
3784:Rivera-Ortiz JM, Burris RH (August 1975).
3779:
3777:
3584:
3532:
2956:
2407:
2204:. Springer Netherlands. pp. 281–307.
2171:. Springer Netherlands. pp. 255–279.
2004:
1264:– Termed the Janus intermediate after the
685:
27:
4108:
4063:
4011:
3904:
3864:
3835:
3826:
3809:
3714:
3638:
3611:
3602:
3585:Cohn, Mildred; Hughes, Thomas R. (1962).
3515:
3474:
3387:
3377:
3328:
3279:
3195:
3135:
3075:
2939:
2851:
2841:
2617:
2599:
2592:Nature Structural & Molecular Biology
2562:
2434:
2352:
2268:
2258:
2167:Hales BJ (2004). "Vanadium Nitrogenase".
2143:
2067:
1995:
757:
663:
4131:
2920:Journal of the American Chemical Society
2881:Journal of the American Chemical Society
2791:Journal of the American Chemical Society
1502:
1333:
1223:
1015:
1002:
994:
830:
559:. These enzymes are responsible for the
4392:7-Hydroxymethyl chlorophyll a reductase
3944:
3774:
3049:
1028:, which is the reduction of nitrogen (N
4877:
3246:Murakami J, Yamaguchi W (2012-05-14).
2436:10.1146/annurev.micro.62.081307.162737
4565:
4258:
3696:
2969:
2166:
1966:
1774:Organisms that synthesize nitrogenase
1457:. Due to the oxidative properties of
1326:Distal and alternating pathways for N
4541:Tetrahydrocannabinolic acid synthase
1918:apparently controlled by the plant.
1089:
1054:
1011:
4087:Raymond J, Siefert JL, Staples CR,
3627:The Journal of Biological Chemistry
3504:The Journal of Biological Chemistry
3463:The Journal of Biological Chemistry
3352:Hageman RV, Burris RH (June 1978).
2381:Current Opinion in Chemical Biology
1894:Measurement of nitrogenase activity
1491:, which can bind to dioxygen via a
1479:, allowing oxygen reduction at the
13:
4198:
3716:10.1111/j.1574-6976.2000.tb00545.x
3303:Dilworth MJ, Eady RR (July 1991).
2193:
2160:
863:pyruvate:ferredoxin oxidoreductase
766:
762:
759:
753:
750:
728:
691:
687:
672:
668:
665:
659:
656:
630:
14:
4911:
4236:
2537:Sippel D, Einsle O (2017-07-10).
1766:through a reaction comparable to
4862:
4551:Dichlorochromopyrrolate synthase
4242:
2093:Metal Ions in Biological Systems
1857:that performs the conversion of
1515:, meaning nitrogenase is also a
1385:
968:) type and the iron–iron (FeFe;
4358:(Methionine synthase) reductase
4160:
4125:
4097:Molecular Biology and Evolution
4080:
3979:
3731:
3690:
3655:
3591:Journal of Biological Chemistry
3578:
3491:
3450:
3345:
3296:
3239:
3212:
2907:
2809:
2782:
2751:
2708:
2579:
2530:
2506:Catalysts for Nitrogen Fixation
2451:
1351:in the distal pathway, and the
1097:+ 14 H + 12 e + 40 MgATP → 2 NH
887:ferredoxin:NADPH oxidoreductase
547:) that are produced by certain
2226:
2111:
2042:Alleman AB, Peters JW (2023).
2035:
1960:
1876:that do not fix nitrogen e.g.
896:
871:photosynthetic reaction center
1:
4529:(-)-bisdechlorogeodin-forming
4524:(+)-bisdechlorogeodin-forming
4177:Soil Biology and Biochemistry
3802:10.1128/JB.123.2.537-545.1975
3640:10.1016/S0021-9258(17)41861-8
3604:10.1016/S0021-9258(18)81382-5
3476:10.1016/S0021-9258(19)50480-X
3056:Accounts of Chemical Research
2694:10.1016/S0301-4622(01)00182-X
2423:Annual Review of Microbiology
1954:
1949:Abiological nitrogen fixation
1879:Methanocaldococcus jannaschii
1865:. This protein is present in
1855:protochlorophyllide reductase
1062:+ 8 H + 8 e + 16 MgATP → 2 NH
955:
609:protochlorophyllide reductase
467:Available protein structures:
280:Available protein structures:
4370:: Acting on CH or CH2 groups
4220:10.1016/0304-4173(75)90012-9
4189:10.1016/0038-0717(84)90118-4
4146:10.1016/0304-4165(66)90383-7
3050:Schrock RR (December 2005).
2514:10.1007/978-1-4020-3611-8_11
2417:Rubio LM, Ludden PW (2008).
2210:10.1007/978-1-4020-3611-8_11
2177:10.1007/978-1-4020-3611-8_10
1849:Similarity to other proteins
1445:Many mechanistic aspects of
1314:signal associated with the E
1142:Lowe-Thorneley kinetic model
990:
843:
615:Classification and structure
7:
4591:
4546:Cannabidiolic acid synthase
4175:reduction) of rice roots".
1937:
1754:rapid-equilibrium inhibitor
1409:exists between residue 15,
1115:
1080:
913:as fully oxidized Mo(VI)).
10:
4916:
2601:10.1038/s41594-023-01124-2
2393:10.1016/j.cbpa.2006.02.019
1782:Free-living bacteria (non-
1381:Mechanism of MgATP binding
555:(blue-green bacteria) and
4748:
4740:Michaelis–Menten kinetics
4712:
4681:
4650:
4599:
4495:
4446:
4423:
4400:
4366:
4338:
4298:
3756:10.1016/j.cub.2005.01.042
3703:FEMS Microbiology Reviews
3026:10.1007/s11244-006-0002-0
2472:10.1007/s11104-008-9833-8
1768:Fischer-Tropsch synthesis
1746:non-competitive inhibitor
1441:Other mechanistic details
1419:Site-specific mutagenesis
1368:to catalytically reduce N
865:, 2. by a bi-directional
789:). Nitrogenase acts as a
509:
489:
471:
466:
462:
450:
438:
430:
425:
420:
404:
384:
372:
360:
352:
347:
342:
322:
302:
284:
279:
275:
255:
243:
231:
223:
218:
213:
193:
181:
169:
164:
160:
141:
129:
117:
105:
93:
81:
69:
64:
52:
40:
35:
26:
21:
4632:Diffusion-limited enzyme
4506:Isopenicillin N synthase
4438:Nitrogenase (flavodoxin)
4382:Ribonucleotide reductase
3697:Oelze J (October 2000).
3517:10.1074/jbc.270.22.13112
1914:Clostridium pasteurianum
1266:Roman god of transitions
1163:, although reaction of E
1038:vanadium (V) nitrogenase
4044:Journal of Bacteriology
3790:Journal of Bacteriology
3564:10.1126/science.1359643
3436:10.1126/science.1529353
3309:The Biochemical Journal
3128:10.1126/science.1211906
2843:10.1073/pnas.0610975104
2737:10.1126/science.6585956
2543:Nature Chemical Biology
2260:10.1073/pnas.1411185111
975:The Anf nitrogenase in
3850:10.1002/anie.197505141
3379:10.1073/pnas.75.6.2699
2984:10.1002/anie.200502667
2136:10.1002/zaac.201400446
2048:Appl Environ Microbiol
1339:
1229:
1021:
1008:
1000:
977:Azotobacter vinelandii
928:Each FeMo cofactor (Fe
877:to dissipation of the
840:
783:
705:
607:. They are related to
4725:Eadie–Hofstee diagram
4658:Allosteric regulation
4110:10.1093/molbev/msh047
4004:10.1105/tpc.5.12.1817
2682:Biophysical Chemistry
2555:10.1038/nchembio.2428
1796:Green sulfur bacteria
1786:), examples include:
1760:Vanadium nitrogenases
1738:competitive inhibitor
1503:Nonspecific reactions
1337:
1290:state. The decay of E
1227:
1150:, freeze quench, and
1019:
1006:
998:
834:
784:
706:
4890:Iron–sulfur proteins
4735:Lineweaver–Burk plot
4427:: Acting on reduced
4406:iron–sulfur proteins
4313:Superoxide dismutase
4251:at Wikimedia Commons
2060:10.1128/aem.00378-23
1902:) to the product (NH
903:iron–sulfur clusters
883:electron bifurcation
719:
627:
621:enthalpy of reaction
4536:Aureusidin synthase
4511:Columbamine oxidase
4056:10.1128/JB.00876-07
3965:10.1021/bi00291a010
3890:10.1021/bi00016a009
3556:1992Sci...258..992W
3428:1992Sci...257.1653G
3370:1978PNAS...75.2699H
3264:2012NatSR...2E.407M
3233:10.1021/cr60316a001
3188:10.1038/nature12435
3180:2013Natur.501...84A
3120:2011Sci...334..780R
3014:Topics in Catalysis
2834:2007PNAS..104.1451L
2760:Dalton Transactions
2729:1984Sci...224.1095S
2251:2014PNAS..111E3718Y
1859:protochlorophyllide
1841:actinorhizal plants
1413:, and residue 125,
979:is organized in an
879:proton motive force
4900:Molybdenum enzymes
4694:Enzyme superfamily
4627:Enzyme promiscuity
4520:Sulochrin oxidase
4516:Reticuline oxidase
3252:Scientific Reports
1981:10.1007/bf02836187
1839:, associated with
1828:, associated with
1817:, associated with
1792:(blue-green algae)
1340:
1310:has confirmed the
1230:
1022:
1009:
1001:
841:
779:
701:
4850:
4849:
4559:
4558:
4247:Media related to
3930:10.1021/bi970217e
3838:Angewandte Chemie
3676:10.1021/bi036038g
3321:10.1042/bj2770465
3272:10.1038/srep00407
3068:10.1021/ar0501121
2972:Angewandte Chemie
2932:10.1021/ja205304t
2893:10.1021/ja043596p
2803:10.1021/ja000254k
2797:(20): 4926–4936.
2656:10.1021/cr950055x
2523:978-90-481-6675-6
2345:10.1021/cr400641x
2219:978-1-4020-3611-8
2186:978-1-4020-3611-8
2124:Z Anorg Allg Chem
2021:10.1021/cr950055x
1944:Nitrogen fixation
1123:
1122:
1105:+ 40 MgADP + 40 P
1088:
1087:
1070:+ 16 MgADP + 16 P
1026:nitrogen fixation
1012:General mechanism
873:, 4. by coupling
835:Structure of the
748:
713:activation energy
654:
581:nitrogen fixation
525:
524:
521:
520:
516:structure summary
416:
415:
338:
337:
334:
333:
329:structure summary
209:
208:
205:
204:
124:metabolic pathway
4907:
4867:
4866:
4858:
4730:Hanes–Woolf plot
4673:Enzyme activator
4668:Enzyme inhibitor
4642:Enzyme catalysis
4586:
4579:
4572:
4563:
4562:
4377:Xanthine oxidase
4279:
4272:
4265:
4256:
4255:
4246:
4231:
4193:
4192:
4164:
4158:
4157:
4129:
4123:
4122:
4112:
4084:
4078:
4077:
4067:
4035:
4026:
4025:
4015:
3983:
3977:
3976:
3948:
3942:
3941:
3913:
3902:
3901:
3873:
3862:
3861:
3833:
3824:
3823:
3813:
3781:
3772:
3771:
3735:
3729:
3728:
3718:
3694:
3688:
3687:
3659:
3653:
3652:
3642:
3618:
3609:
3608:
3606:
3582:
3576:
3575:
3539:
3530:
3529:
3519:
3495:
3489:
3488:
3478:
3454:
3448:
3447:
3422:(5077): 1653–9.
3411:
3402:
3401:
3391:
3381:
3349:
3343:
3342:
3332:
3300:
3294:
3293:
3283:
3243:
3237:
3236:
3216:
3210:
3209:
3199:
3159:
3150:
3149:
3139:
3099:
3090:
3089:
3079:
3047:
3038:
3037:
3009:
2996:
2995:
2967:
2954:
2953:
2943:
2926:(43): 17329–40.
2911:
2905:
2904:
2875:
2866:
2865:
2855:
2845:
2813:
2807:
2806:
2786:
2780:
2779:
2768:10.1039/B517633F
2755:
2749:
2748:
2723:(4653): 1095–7.
2712:
2706:
2705:
2677:
2668:
2667:
2650:(7): 2983–3012.
2644:Chemical Reviews
2639:
2624:
2623:
2621:
2603:
2583:
2577:
2576:
2566:
2534:
2528:
2527:
2501:
2492:
2491:
2455:
2449:
2448:
2438:
2414:
2405:
2404:
2376:
2367:
2366:
2356:
2333:Chemical Reviews
2324:
2283:
2282:
2272:
2262:
2245:(35): E3718-25.
2230:
2224:
2223:
2197:
2191:
2190:
2164:
2158:
2157:
2147:
2115:
2109:
2108:
2088:
2082:
2081:
2071:
2054:(5): e00378-23.
2039:
2033:
2032:
2015:(7): 2983–3011.
2008:Chemical Reviews
2002:
1993:
1992:
1964:
1756:of nitrogenase.
1750:carbon disulfide
1576:
1575:
1574:
1473:Azotobacteraceae
1435:X-ray scattering
1396:of the terminal
1389:
1302:and finally to E
1117:
1090:
1082:
1055:
1032:) to ammonia (NH
940:residue and one
817:heterotetrameric
788:
786:
785:
780:
778:
777:
776:
756:
746:
733:
732:
731:
710:
708:
707:
702:
700:
699:
698:
684:
683:
682:
662:
652:
642:
641:
464:
463:
418:
417:
340:
339:
277:
276:
211:
210:
162:
161:
31:
19:
18:
16:Class of enzymes
4915:
4914:
4910:
4909:
4908:
4906:
4905:
4904:
4875:
4874:
4873:
4861:
4853:
4851:
4846:
4758:Oxidoreductases
4744:
4720:Enzyme kinetics
4708:
4704:List of enzymes
4677:
4646:
4617:Catalytic triad
4595:
4590:
4560:
4555:
4491:
4442:
4419:
4396:
4362:
4334:
4294:
4287:oxidoreductases
4283:
4239:
4234:
4201:
4199:Further reading
4196:
4174:
4170:
4165:
4161:
4130:
4126:
4085:
4081:
4036:
4029:
3998:(12): 1817–29.
3984:
3980:
3959:(22): 5111–22.
3949:
3945:
3924:(28): 8574–85.
3914:
3905:
3874:
3865:
3834:
3827:
3782:
3775:
3744:Current Biology
3736:
3732:
3695:
3691:
3660:
3656:
3619:
3612:
3583:
3579:
3550:(5084): 992–5.
3540:
3533:
3510:(22): 13112–7.
3496:
3492:
3455:
3451:
3412:
3405:
3364:(6): 2699–702.
3350:
3346:
3301:
3297:
3244:
3240:
3217:
3213:
3160:
3153:
3114:(6057): 780–3.
3100:
3093:
3048:
3041:
3010:
2999:
2968:
2957:
2912:
2908:
2887:(17): 6231–41.
2876:
2869:
2814:
2810:
2787:
2783:
2762:(19): 2277–84.
2756:
2752:
2713:
2709:
2678:
2671:
2640:
2627:
2584:
2580:
2535:
2531:
2524:
2502:
2495:
2456:
2452:
2415:
2408:
2377:
2370:
2325:
2286:
2231:
2227:
2220:
2198:
2194:
2187:
2165:
2161:
2116:
2112:
2089:
2085:
2040:
2036:
2003:
1996:
1965:
1961:
1957:
1940:
1932:
1929:competes with N
1928:
1924:
1909:
1905:
1901:
1896:
1851:
1776:
1752:functions as a
1744:functions as a
1742:carbon monoxide
1736:functions as a
1728:
1720:
1710:
1700:
1689:
1674:
1669:
1665:
1658:
1654:
1648:
1644:
1640:
1636:
1632:
1626:
1622:
1615:
1611:
1607:
1603:
1598:
1594:
1588:
1583:
1573:
1571:
1570:
1569:
1567:
1563:
1559:
1549:
1545:
1535:
1531:
1505:
1498:
1486:
1443:
1383:
1371:
1345:
1332:
1329:
1317:
1309:
1305:
1301:
1297:
1293:
1289:
1271:
1262:
1253:
1245:
1236:
1216:
1211:
1203:
1194:
1184:
1182:
1178:
1175:Intermediates E
1170:
1166:
1162:
1158:
1144:
1137:
1133:
1108:
1104:
1100:
1096:
1073:
1069:
1065:
1061:
1035:
1031:
1014:
993:
958:
951:
935:
931:
924:
920:
899:
846:
826:
822:
769:
765:
758:
749:
727:
726:
722:
720:
717:
716:
694:
690:
686:
675:
671:
664:
655:
637:
633:
628:
625:
624:
617:
578:
570:
17:
12:
11:
5:
4913:
4903:
4902:
4897:
4895:Nitrogen cycle
4892:
4887:
4872:
4871:
4848:
4847:
4845:
4844:
4831:
4818:
4805:
4792:
4779:
4766:
4752:
4750:
4746:
4745:
4743:
4742:
4737:
4732:
4727:
4722:
4716:
4714:
4710:
4709:
4707:
4706:
4701:
4696:
4691:
4685:
4683:
4682:Classification
4679:
4678:
4676:
4675:
4670:
4665:
4660:
4654:
4652:
4648:
4647:
4645:
4644:
4639:
4634:
4629:
4624:
4619:
4614:
4609:
4603:
4601:
4597:
4596:
4589:
4588:
4581:
4574:
4566:
4557:
4556:
4554:
4553:
4548:
4543:
4538:
4533:
4532:
4531:
4526:
4518:
4513:
4508:
4502:
4500:
4493:
4492:
4490:
4489:
4488:
4487:
4482:
4477:
4472:
4461:
4459:
4444:
4443:
4441:
4440:
4434:
4432:
4421:
4420:
4418:
4417:
4411:
4409:
4398:
4397:
4395:
4394:
4389:
4384:
4379:
4373:
4371:
4364:
4363:
4361:
4360:
4355:
4349:
4347:
4336:
4335:
4333:
4332:
4331:
4330:
4325:
4320:
4309:
4307:
4296:
4295:
4282:
4281:
4274:
4267:
4259:
4253:
4252:
4238:
4237:External links
4235:
4233:
4232:
4202:
4200:
4197:
4195:
4194:
4172:
4168:
4159:
4124:
4091:(March 2004).
4089:Blankenship RE
4079:
4050:(20): 7392–8.
4027:
3992:The Plant Cell
3978:
3943:
3903:
3884:(16): 5382–9.
3863:
3825:
3773:
3750:(6): 531–535.
3730:
3689:
3654:
3610:
3577:
3531:
3490:
3469:(10): 6680–8.
3449:
3403:
3344:
3295:
3238:
3227:(6): 589–625.
3211:
3174:(7465): 84–7.
3151:
3091:
3062:(12): 955–62.
3039:
2997:
2955:
2906:
2867:
2808:
2781:
2750:
2707:
2688:(3): 281–304.
2669:
2625:
2578:
2549:(9): 956–960.
2529:
2522:
2493:
2466:(1–2): 35–59.
2460:Plant and Soil
2450:
2406:
2368:
2339:(8): 4041–62.
2284:
2225:
2218:
2192:
2185:
2159:
2110:
2083:
2034:
1994:
1958:
1956:
1953:
1952:
1951:
1946:
1939:
1936:
1930:
1926:
1922:
1907:
1903:
1899:
1895:
1892:
1850:
1847:
1846:
1845:
1844:
1843:
1832:
1821:
1807:
1806:
1805:
1798:
1793:
1775:
1772:
1730:
1729:
1726:
1718:
1712:
1708:
1702:
1698:
1692:
1687:
1675:
1672:
1667:
1663:
1656:
1652:
1646:
1642:
1638:
1634:
1630:
1627:
1624:
1620:
1617:
1613:
1609:
1605:
1601:
1596:
1592:
1586:
1581:
1572:
1564:
1561:
1557:
1551:
1547:
1543:
1537:
1533:
1529:
1504:
1501:
1496:
1484:
1477:metabolic rate
1442:
1439:
1382:
1379:
1369:
1343:
1331:
1327:
1324:
1318:intermediate.
1315:
1307:
1303:
1299:
1295:
1291:
1287:
1269:
1260:
1251:
1243:
1234:
1214:
1209:
1201:
1192:
1183:
1180:
1176:
1173:
1168:
1164:
1160:
1156:
1143:
1140:
1135:
1131:
1121:
1120:
1111:
1109:
1106:
1102:
1098:
1094:
1086:
1085:
1076:
1074:
1071:
1067:
1063:
1059:
1033:
1029:
1013:
1010:
992:
989:
957:
954:
949:
946:
945:
933:
929:
926:
922:
918:
907:FeMo cofactors
898:
895:
851:reducing agent
845:
842:
829:
828:
824:
820:
813:
775:
772:
768:
764:
761:
755:
752:
745:
742:
739:
736:
730:
725:
715:is very high (
697:
693:
689:
681:
678:
674:
670:
667:
661:
658:
651:
648:
645:
640:
636:
632:
616:
613:
576:
568:
523:
522:
519:
518:
513:
507:
506:
493:
487:
486:
476:
469:
468:
460:
459:
454:
448:
447:
442:
436:
435:
432:
428:
427:
423:
422:
414:
413:
408:
402:
401:
388:
382:
381:
376:
370:
369:
364:
358:
357:
354:
350:
349:
345:
344:
336:
335:
332:
331:
326:
320:
319:
306:
300:
299:
289:
282:
281:
273:
272:
259:
253:
252:
247:
241:
240:
235:
229:
228:
227:Oxidored_nitro
225:
221:
220:
216:
215:
207:
206:
203:
202:
197:
191:
190:
185:
179:
178:
173:
167:
166:
158:
157:
146:
139:
138:
133:
127:
126:
121:
115:
114:
109:
103:
102:
97:
91:
90:
85:
79:
78:
73:
67:
66:
62:
61:
56:
50:
49:
44:
38:
37:
33:
32:
24:
23:
15:
9:
6:
4:
3:
2:
4912:
4901:
4898:
4896:
4893:
4891:
4888:
4886:
4883:
4882:
4880:
4870:
4865:
4860:
4859:
4856:
4842:
4838:
4837:
4832:
4829:
4825:
4824:
4819:
4816:
4812:
4811:
4806:
4803:
4799:
4798:
4793:
4790:
4786:
4785:
4780:
4777:
4773:
4772:
4767:
4764:
4760:
4759:
4754:
4753:
4751:
4747:
4741:
4738:
4736:
4733:
4731:
4728:
4726:
4723:
4721:
4718:
4717:
4715:
4711:
4705:
4702:
4700:
4699:Enzyme family
4697:
4695:
4692:
4690:
4687:
4686:
4684:
4680:
4674:
4671:
4669:
4666:
4664:
4663:Cooperativity
4661:
4659:
4656:
4655:
4653:
4649:
4643:
4640:
4638:
4635:
4633:
4630:
4628:
4625:
4623:
4622:Oxyanion hole
4620:
4618:
4615:
4613:
4610:
4608:
4605:
4604:
4602:
4598:
4594:
4587:
4582:
4580:
4575:
4573:
4568:
4567:
4564:
4552:
4549:
4547:
4544:
4542:
4539:
4537:
4534:
4530:
4527:
4525:
4522:
4521:
4519:
4517:
4514:
4512:
4509:
4507:
4504:
4503:
4501:
4498:
4494:
4486:
4483:
4481:
4478:
4476:
4473:
4471:
4468:
4467:
4466:
4463:
4462:
4460:
4457:
4453:
4449:
4445:
4439:
4436:
4435:
4433:
4430:
4426:
4422:
4416:
4413:
4412:
4410:
4407:
4403:
4399:
4393:
4390:
4388:
4385:
4383:
4380:
4378:
4375:
4374:
4372:
4369:
4365:
4359:
4356:
4354:
4353:Ceruloplasmin
4351:
4350:
4348:
4345:
4341:
4337:
4329:
4326:
4324:
4321:
4319:
4316:
4315:
4314:
4311:
4310:
4308:
4305:
4301:
4297:
4292:
4288:
4280:
4275:
4273:
4268:
4266:
4261:
4260:
4257:
4250:
4245:
4241:
4240:
4229:
4225:
4221:
4217:
4213:
4209:
4204:
4203:
4190:
4186:
4182:
4178:
4163:
4155:
4151:
4147:
4143:
4140:(2): 285–94.
4139:
4135:
4128:
4120:
4116:
4111:
4106:
4103:(3): 541–54.
4102:
4098:
4094:
4090:
4083:
4075:
4071:
4066:
4061:
4057:
4053:
4049:
4045:
4041:
4034:
4032:
4023:
4019:
4014:
4009:
4005:
4001:
3997:
3993:
3989:
3982:
3974:
3970:
3966:
3962:
3958:
3954:
3947:
3939:
3935:
3931:
3927:
3923:
3919:
3912:
3910:
3908:
3899:
3895:
3891:
3887:
3883:
3879:
3872:
3870:
3868:
3859:
3855:
3851:
3847:
3844:(8): 514–22.
3843:
3839:
3832:
3830:
3821:
3817:
3812:
3807:
3803:
3799:
3796:(2): 537–45.
3795:
3791:
3787:
3780:
3778:
3770:
3765:
3761:
3757:
3753:
3749:
3745:
3741:
3734:
3726:
3722:
3717:
3712:
3709:(4): 321–33.
3708:
3704:
3700:
3693:
3685:
3681:
3677:
3673:
3670:(6): 1401–9.
3669:
3665:
3658:
3650:
3646:
3641:
3636:
3633:(5): 3290–4.
3632:
3628:
3624:
3617:
3615:
3605:
3600:
3596:
3592:
3588:
3581:
3573:
3569:
3565:
3561:
3557:
3553:
3549:
3545:
3538:
3536:
3527:
3523:
3518:
3513:
3509:
3505:
3501:
3494:
3486:
3482:
3477:
3472:
3468:
3464:
3460:
3453:
3445:
3441:
3437:
3433:
3429:
3425:
3421:
3417:
3410:
3408:
3399:
3395:
3390:
3385:
3380:
3375:
3371:
3367:
3363:
3359:
3355:
3348:
3340:
3336:
3331:
3326:
3322:
3318:
3314:
3310:
3306:
3299:
3291:
3287:
3282:
3277:
3273:
3269:
3265:
3261:
3257:
3253:
3249:
3242:
3234:
3230:
3226:
3222:
3215:
3207:
3203:
3198:
3193:
3189:
3185:
3181:
3177:
3173:
3169:
3165:
3158:
3156:
3147:
3143:
3138:
3133:
3129:
3125:
3121:
3117:
3113:
3109:
3105:
3098:
3096:
3087:
3083:
3078:
3073:
3069:
3065:
3061:
3057:
3053:
3046:
3044:
3035:
3031:
3027:
3023:
3019:
3015:
3008:
3006:
3004:
3002:
2993:
2989:
2985:
2981:
2977:
2973:
2966:
2964:
2962:
2960:
2951:
2947:
2942:
2937:
2933:
2929:
2925:
2921:
2917:
2910:
2902:
2898:
2894:
2890:
2886:
2882:
2874:
2872:
2863:
2859:
2854:
2849:
2844:
2839:
2835:
2831:
2828:(5): 1451–5.
2827:
2823:
2819:
2812:
2804:
2800:
2796:
2792:
2785:
2777:
2773:
2769:
2765:
2761:
2754:
2746:
2742:
2738:
2734:
2730:
2726:
2722:
2718:
2711:
2703:
2699:
2695:
2691:
2687:
2683:
2676:
2674:
2665:
2661:
2657:
2653:
2649:
2645:
2638:
2636:
2634:
2632:
2630:
2620:
2615:
2611:
2607:
2602:
2597:
2593:
2589:
2582:
2574:
2570:
2565:
2560:
2556:
2552:
2548:
2544:
2540:
2533:
2525:
2519:
2515:
2511:
2507:
2500:
2498:
2489:
2485:
2481:
2477:
2473:
2469:
2465:
2461:
2454:
2446:
2442:
2437:
2432:
2429:(1): 93–111.
2428:
2424:
2420:
2413:
2411:
2402:
2398:
2394:
2390:
2386:
2382:
2375:
2373:
2364:
2360:
2355:
2350:
2346:
2342:
2338:
2334:
2330:
2323:
2321:
2319:
2317:
2315:
2313:
2311:
2309:
2307:
2305:
2303:
2301:
2299:
2297:
2295:
2293:
2291:
2289:
2280:
2276:
2271:
2266:
2261:
2256:
2252:
2248:
2244:
2240:
2236:
2229:
2221:
2215:
2211:
2207:
2203:
2196:
2188:
2182:
2178:
2174:
2170:
2163:
2155:
2151:
2146:
2141:
2137:
2133:
2129:
2125:
2121:
2114:
2106:
2102:
2098:
2094:
2087:
2079:
2075:
2070:
2065:
2061:
2057:
2053:
2049:
2045:
2038:
2030:
2026:
2022:
2018:
2014:
2010:
2009:
2001:
1999:
1990:
1986:
1982:
1978:
1974:
1970:
1963:
1959:
1950:
1947:
1945:
1942:
1941:
1935:
1919:
1916:
1915:
1891:
1889:
1888:M. jannaschii
1885:
1881:
1880:
1875:
1870:
1868:
1864:
1860:
1856:
1842:
1838:
1837:
1833:
1831:
1827:
1826:
1822:
1820:
1816:
1815:
1811:
1810:
1808:
1804:
1803:
1799:
1797:
1794:
1791:
1790:Cyanobacteria
1788:
1787:
1785:
1781:
1780:
1779:
1771:
1769:
1765:
1761:
1757:
1755:
1751:
1747:
1743:
1739:
1735:
1732:Furthermore,
1724:
1716:
1713:
1706:
1703:
1696:
1693:
1691:
1683:
1679:
1676:
1670:
1659:
1628:
1618:
1599:
1584:
1577:
1565:
1555:
1552:
1541:
1538:
1536:
1525:
1522:
1521:
1520:
1518:
1517:dehydrogenase
1514:
1510:
1500:
1494:
1490:
1489:leghemoglobin
1482:
1481:cell membrane
1478:
1474:
1470:
1469:
1464:
1460:
1456:
1450:
1448:
1438:
1436:
1432:
1431:glutamic acid
1428:
1424:
1420:
1416:
1415:aspartic acid
1412:
1408:
1403:
1399:
1395:
1390:
1388:
1378:
1375:
1365:
1363:
1358:
1354:
1350:
1336:
1323:
1319:
1313:
1284:
1280:
1276:
1267:
1263:
1256:
1254:
1247:
1241:
1237:
1226:
1222:
1220:
1212:
1205:
1199:
1195:
1188:
1172:
1153:
1149:
1139:
1138:takes place.
1129:
1128:FeMo-cofactor
1119:
1112:
1110:
1092:
1091:
1084:
1077:
1075:
1057:
1056:
1053:
1051:
1047:
1043:
1039:
1027:
1018:
1005:
997:
988:
986:
982:
978:
973:
971:
967:
963:
962:vanadium–iron
953:
943:
939:
927:
916:
915:
914:
912:
911:molybdopterin
908:
904:
894:
892:
888:
885:, or 6. by a
884:
880:
876:
875:electron flow
872:
868:
864:
860:
856:
852:
838:
837:FeMo cofactor
833:
818:
814:
811:
807:
803:
799:
798:
797:
794:
792:
773:
770:
743:
740:
737:
734:
723:
714:
695:
679:
676:
649:
646:
643:
638:
634:
622:
612:
610:
606:
602:
598:
594:
590:
586:
582:
574:
566:
562:
558:
557:rhizobacteria
554:
553:cyanobacteria
550:
546:
543:
540:
537:
533:
529:
517:
514:
512:
508:
505:
501:
497:
494:
492:
488:
484:
480:
477:
474:
470:
465:
461:
458:
455:
453:
449:
446:
443:
441:
437:
433:
429:
424:
419:
412:
409:
407:
403:
400:
396:
392:
389:
387:
383:
380:
377:
375:
371:
368:
365:
363:
359:
355:
351:
346:
341:
330:
327:
325:
321:
318:
314:
310:
307:
305:
301:
297:
293:
290:
287:
283:
278:
274:
271:
267:
263:
260:
258:
254:
251:
248:
246:
242:
239:
236:
234:
230:
226:
222:
217:
212:
201:
198:
196:
192:
189:
186:
184:
180:
177:
174:
172:
168:
163:
159:
156:
153:
150:
147:
144:
140:
137:
134:
132:
128:
125:
122:
120:
116:
113:
110:
108:
104:
101:
100:NiceZyme view
98:
96:
92:
89:
86:
84:
80:
77:
74:
72:
68:
63:
60:
57:
55:
51:
48:
45:
43:
39:
34:
30:
25:
20:
4836:Translocases
4833:
4820:
4807:
4794:
4781:
4771:Transferases
4768:
4755:
4612:Binding site
4465:Glutaredoxin
4450:: Acting on
4414:
4404:: Acting on
4342:: Oxidizing
4302:: Acting on
4211:
4207:
4180:
4176:
4162:
4137:
4133:
4127:
4100:
4096:
4082:
4047:
4043:
3995:
3991:
3981:
3956:
3953:Biochemistry
3952:
3946:
3921:
3918:Biochemistry
3917:
3881:
3878:Biochemistry
3877:
3841:
3837:
3793:
3789:
3767:
3747:
3743:
3733:
3706:
3702:
3692:
3667:
3664:Biochemistry
3663:
3657:
3630:
3626:
3594:
3590:
3580:
3547:
3543:
3507:
3503:
3493:
3466:
3462:
3452:
3419:
3415:
3361:
3357:
3347:
3315:(2): 465–8.
3312:
3308:
3298:
3255:
3251:
3241:
3224:
3220:
3214:
3171:
3167:
3111:
3107:
3059:
3055:
3020:(1): 55–70.
3017:
3013:
2978:(2): 196–9.
2975:
2971:
2923:
2919:
2909:
2884:
2880:
2825:
2821:
2811:
2794:
2790:
2784:
2759:
2753:
2720:
2716:
2710:
2685:
2681:
2647:
2643:
2591:
2581:
2546:
2542:
2532:
2505:
2463:
2459:
2453:
2426:
2422:
2387:(2): 101–8.
2384:
2380:
2336:
2332:
2242:
2238:
2228:
2201:
2195:
2168:
2162:
2130:(1): 65–71.
2127:
2123:
2113:
2096:
2092:
2086:
2051:
2047:
2037:
2012:
2006:
1975:(9): 69–77.
1972:
1968:
1962:
1920:
1912:
1906:). Since NH
1897:
1887:
1883:
1877:
1871:
1852:
1834:
1825:Azospirillum
1823:
1812:
1800:
1777:
1758:
1731:
1506:
1466:
1451:
1444:
1391:
1384:
1366:
1341:
1320:
1258:
1257:
1249:
1248:
1232:
1231:
1207:
1206:
1190:
1189:
1185:
1152:stopped-flow
1148:Steady state
1145:
1124:
1113:
1078:
1048:-associated
1023:
980:
976:
974:
969:
965:
959:
947:
917:The core (Fe
900:
847:
795:
618:
585:biosynthesis
528:Nitrogenases
527:
526:
88:BRENDA entry
4607:Active site
4415:Nitrogenase
4306:as acceptor
4249:Nitrogenase
4214:(1): 1–52.
3597:: 176–181.
1874:methanogens
1867:gymnosperms
1863:chlorophyll
1802:Azotobacter
1619:N≡C–R → RCH
1407:salt bridge
1042:diazotrophs
897:Nitrogenase
867:hydrogenase
802:homodimeric
597:amino acids
593:nucleotides
426:Identifiers
348:Identifiers
219:Identifiers
76:IntEnz view
36:Identifiers
22:Nitrogenase
4879:Categories
4810:Isomerases
4784:Hydrolases
4651:Regulation
4452:phosphorus
4429:flavodoxin
4304:superoxide
4293:1.15–1.21)
2099:: 75–119.
1955:References
1734:dihydrogen
1629:C≡N–R → CH
1513:dihydrogen
1402:amino acid
1394:Hydrolysis
1187:nitrogen:
956:Variations
869:, 3. in a
859:flavodoxin
855:ferredoxin
853:, such as
551:, such as
479:structures
292:structures
145:structures
112:KEGG entry
59:9013-04-1
4885:EC 1.18.6
4689:EC number
4458:in donors
4408:as donors
4183:: 15–18.
3764:0960-9822
3221:Chem. Rev
2610:1545-9985
2480:0032-079X
1989:195305228
1969:Resonance
1814:Rhizobium
1784:symbiotic
1725:, CO + NH
1447:catalysis
1423:glutamine
1398:phosphate
1357:hydrazine
1219:Mӧssbauer
1179:through E
991:Mechanism
985:Nif genes
981:anfHDGKOR
942:histidine
844:Reductase
810:reductase
771:−
741:−
677:−
647:−
631:Δ
601:Nif genes
589:molecules
561:reduction
457:IPR004349
434:AnfG_VnfG
367:IPR005977
250:IPR000510
65:Databases
4713:Kinetics
4637:Cofactor
4600:Activity
4431:as donor
4119:14694078
4074:17660283
3725:10978541
3684:14769015
3290:22586517
3206:24005414
3146:22076372
3086:16359167
3034:93357657
2992:16342309
2950:21980917
2901:15853328
2862:17251348
2776:16688314
2702:11551440
2664:11848849
2619:10803253
2573:28692069
2488:10892514
2445:18429691
2401:16510305
2363:24467365
2279:25139995
2154:26213424
2105:11913144
2078:37154716
2069:10231201
2029:11848849
1938:See also
1711:S + HCN
1697:→ CO + H
1463:dioxygen
1455:ethylene
1427:arginine
1374:tungsten
1372:. Small
1330:fixation
1050:rhizobia
1044:such as
944:residue.
938:cysteine
881:, 5. by
804:Fe-only
791:catalyst
605:homologs
565:nitrogen
549:bacteria
545:1.19.6.1
539:1.18.6.1
496:RCSB PDB
452:InterPro
362:InterPro
309:RCSB PDB
245:InterPro
200:proteins
188:articles
176:articles
149:RCSB PDB
47:1.18.6.1
4869:Biology
4823:Ligases
4593:Enzymes
4456:arsenic
4154:5964974
4065:2168459
4022:8305874
3973:6360203
3938:9214303
3898:7727396
3820:1150625
3649:8106367
3572:1359643
3552:Bibcode
3544:Science
3526:7768906
3485:1313018
3444:1529353
3424:Bibcode
3416:Science
3366:Bibcode
3339:1859374
3330:1151257
3281:3350986
3260:Bibcode
3258:: 407.
3197:3882122
3176:Bibcode
3137:3218428
3116:Bibcode
3108:Science
3077:2551323
2941:3232045
2853:1785236
2830:Bibcode
2745:6585956
2725:Bibcode
2717:Science
2594:: 1–9.
2564:5563456
2354:4012840
2270:4156695
2247:Bibcode
2145:4510703
1836:Frankia
1830:grasses
1819:legumes
1764:alkanes
1509:protons
1468:in vivo
1353:diazene
1349:nitrido
1240:hydride
806:protein
711:), the
573:ammonia
532:enzymes
445:PF03139
411:cd02040
391:d1fp6a_
238:PF00148
136:profile
119:MetaCyc
54:CAS no.
4855:Portal
4797:Lyases
4285:Other
4228:164247
4226:
4152:
4117:
4072:
4062:
4020:
4013:160407
4010:
3971:
3936:
3896:
3858:810048
3856:
3818:
3811:235759
3808:
3762:
3723:
3682:
3647:
3570:
3524:
3483:
3442:
3398:275837
3396:
3389:392630
3386:
3337:
3327:
3288:
3278:
3204:
3194:
3168:Nature
3144:
3134:
3084:
3074:
3032:
2990:
2948:
2938:
2899:
2860:
2850:
2774:
2743:
2700:
2662:
2616:
2608:
2571:
2561:
2520:
2486:
2478:
2443:
2399:
2361:
2351:
2277:
2267:
2216:
2183:
2152:
2142:
2103:
2076:
2066:
2027:
1987:
1748:, and
1459:oxygen
1411:lysine
1306:and 2H
1167:with N
1046:legume
964:(VFe;
808:, the
747:
653:
511:PDBsum
485:
475:
431:Symbol
399:SUPFAM
353:Symbol
324:PDBsum
298:
288:
270:SUPFAM
224:Symbol
183:PubMed
165:Search
155:PDBsum
95:ExPASy
83:BRENDA
71:IntEnz
42:EC no.
4749:Types
4485:GLRX5
4480:GLRX3
4475:GLRX2
4344:metal
3030:S2CID
2484:S2CID
1985:S2CID
1715:O=C=N
1705:S=C=N
1695:O=C=O
1678:O=C=S
1671:, RNH
1554:N=N=N
1540:N=N=O
1524:HC≡CH
1283:ENDOR
1279:ENDOR
1134:to NH
1101:+ 3 H
823:to NH
571:) to
395:SCOPe
386:SCOP2
266:SCOPe
257:SCOP2
131:PRIAM
4841:list
4834:EC7
4828:list
4821:EC6
4815:list
4808:EC5
4802:list
4795:EC4
4789:list
4782:EC3
4776:list
4769:EC2
4763:list
4756:EC1
4497:1.21
4470:GLRX
4448:1.20
4425:1.19
4402:1.18
4368:1.17
4346:ions
4340:1.16
4328:SOD3
4323:SOD2
4318:SOD1
4300:1.15
4224:PMID
4150:PMID
4115:PMID
4070:PMID
4018:PMID
3969:PMID
3934:PMID
3894:PMID
3854:PMID
3816:PMID
3769:SNF.
3760:ISSN
3721:PMID
3680:PMID
3645:PMID
3568:PMID
3522:PMID
3481:PMID
3440:PMID
3394:PMID
3335:PMID
3286:PMID
3202:PMID
3142:PMID
3082:PMID
2988:PMID
2946:PMID
2897:PMID
2858:PMID
2772:PMID
2741:PMID
2698:PMID
2660:PMID
2606:ISSN
2569:PMID
2518:ISBN
2476:ISSN
2441:PMID
2397:PMID
2359:PMID
2275:PMID
2214:ISBN
2181:ISBN
2150:PMID
2101:PMID
2074:PMID
2025:PMID
1721:O +
1645:C=CH
1637:C–CH
1623:+ NH
1604:C=CH
1595:C–CH
1585:, NH
1560:+ NH
1532:C=CH
1493:heme
1355:and
1294:to E
815:The
800:The
650:45.2
530:are
504:PDBj
500:PDBe
483:ECOD
473:Pfam
440:Pfam
379:1fp6
374:CATH
356:NifH
317:PDBj
313:PDBe
296:ECOD
286:Pfam
262:1mio
233:Pfam
195:NCBI
152:PDBe
107:KEGG
4454:or
4216:doi
4212:416
4185:doi
4142:doi
4138:127
4105:doi
4060:PMC
4052:doi
4048:189
4008:PMC
4000:doi
3961:doi
3926:doi
3886:doi
3846:doi
3806:PMC
3798:doi
3794:123
3752:doi
3711:doi
3672:doi
3635:doi
3631:269
3599:doi
3595:237
3560:doi
3548:258
3512:doi
3508:270
3471:doi
3467:267
3432:doi
3420:257
3384:PMC
3374:doi
3325:PMC
3317:doi
3313:277
3276:PMC
3268:doi
3229:doi
3192:PMC
3184:doi
3172:501
3132:PMC
3124:doi
3112:334
3072:PMC
3064:doi
3022:doi
2980:doi
2936:PMC
2928:doi
2924:133
2889:doi
2885:127
2848:PMC
2838:doi
2826:104
2799:doi
2795:122
2764:doi
2733:doi
2721:224
2690:doi
2652:doi
2614:PMC
2596:doi
2559:PMC
2551:doi
2510:doi
2468:doi
2464:321
2431:doi
2389:doi
2349:PMC
2341:doi
2337:114
2265:PMC
2255:doi
2243:111
2206:doi
2173:doi
2140:PMC
2132:doi
2128:641
2064:PMC
2056:doi
2017:doi
1977:doi
1884:nif
1861:to
1723:HCN
1717:→ H
1707:→ H
1641:, H
1633:, H
1608:(CH
1600:, H
1568:C≡N
1556:→ N
1546:+ H
1542:→ N
1511:to
1312:EPR
1298:+ H
1275:EPR
1198:EPR
1066:+ H
970:Anf
966:Vnf
932:MoS
891:ATP
857:or
744:420
738:230
603:or
587:of
575:(NH
563:of
491:PDB
406:CDD
304:PDB
171:PMC
143:PDB
4881::
4291:EC
4222:.
4210:.
4181:16
4179:.
4148:.
4136:.
4113:.
4101:21
4099:.
4095:.
4068:.
4058:.
4046:.
4042:.
4030:^
4016:.
4006:.
3994:.
3990:.
3967:.
3957:22
3955:.
3932:.
3922:36
3920:.
3906:^
3892:.
3882:34
3880:.
3866:^
3852:.
3842:14
3840:.
3828:^
3814:.
3804:.
3792:.
3788:.
3776:^
3766:.
3758:.
3748:15
3746:.
3742:.
3719:.
3707:24
3705:.
3701:.
3678:.
3668:43
3666:.
3643:.
3629:.
3625:.
3613:^
3593:.
3589:.
3566:.
3558:.
3546:.
3534:^
3520:.
3506:.
3502:.
3479:.
3465:.
3461:.
3438:.
3430:.
3418:.
3406:^
3392:.
3382:.
3372:.
3362:75
3360:.
3356:.
3333:.
3323:.
3311:.
3307:.
3284:.
3274:.
3266:.
3254:.
3250:.
3225:78
3223:.
3200:.
3190:.
3182:.
3170:.
3166:.
3154:^
3140:.
3130:.
3122:.
3110:.
3106:.
3094:^
3080:.
3070:.
3060:38
3058:.
3054:.
3042:^
3028:.
3018:37
3016:.
3000:^
2986:.
2976:45
2974:.
2958:^
2944:.
2934:.
2922:.
2918:.
2895:.
2883:.
2870:^
2856:.
2846:.
2836:.
2824:.
2820:.
2793:.
2770:.
2739:.
2731:.
2719:.
2696:.
2686:91
2684:.
2672:^
2658:.
2648:96
2646:.
2628:^
2612:.
2604:.
2590:.
2567:.
2557:.
2547:13
2545:.
2541:.
2516:.
2496:^
2482:.
2474:.
2462:.
2439:.
2427:62
2425:.
2421:.
2409:^
2395:.
2385:10
2371:^
2357:.
2347:.
2335:.
2331:.
2287:^
2273:.
2263:.
2253:.
2241:.
2237:.
2212:.
2179:.
2148:.
2138:.
2126:.
2122:.
2097:39
2095:.
2072:.
2062:.
2052:89
2050:.
2046:.
2023:.
2013:96
2011:.
1997:^
1983:.
1971:.
1770:.
1740:,
1701:O
1684:+
1682:CO
1680:→
1660:,
1649:,
1612:NH
1589:,
1580:CH
1578:→
1526:→
1362:Mo
1246:.
1217:.
1204:.
611:.
595:,
567:(N
542:EC
536:EC
502:;
498:;
481:/
397:/
393:/
315:;
311:;
294:/
268:/
264:/
4857::
4843:)
4839:(
4830:)
4826:(
4817:)
4813:(
4804:)
4800:(
4791:)
4787:(
4778:)
4774:(
4765:)
4761:(
4585:e
4578:t
4571:v
4289:(
4278:e
4271:t
4264:v
4230:.
4218::
4191:.
4187::
4173:2
4171:H
4169:2
4156:.
4144::
4121:.
4107::
4076:.
4054::
4024:.
4002::
3996:5
3975:.
3963::
3940:.
3928::
3900:.
3888::
3860:.
3848::
3822:.
3800::
3754::
3727:.
3713::
3686:.
3674::
3651:.
3637::
3607:.
3601::
3574:.
3562::
3554::
3528:.
3514::
3487:.
3473::
3446:.
3434::
3426::
3400:.
3376::
3368::
3341:.
3319::
3292:.
3270::
3262::
3256:2
3235:.
3231::
3208:.
3186::
3178::
3148:.
3126::
3118::
3088:.
3066::
3036:.
3024::
2994:.
2982::
2952:.
2930::
2903:.
2891::
2864:.
2840::
2832::
2805:.
2801::
2778:.
2766::
2747:.
2735::
2727::
2704:.
2692::
2666:.
2654::
2622:.
2598::
2575:.
2553::
2526:.
2512::
2490:.
2470::
2447:.
2433::
2403:.
2391::
2365:.
2343::
2281:.
2257::
2249::
2222:.
2208::
2189:.
2175::
2156:.
2134::
2107:.
2080:.
2058::
2031:.
2019::
1991:.
1979::
1973:7
1931:2
1927:2
1923:2
1908:3
1904:3
1900:2
1727:3
1719:2
1709:2
1699:2
1690:S
1688:2
1686:H
1673:2
1668:6
1666:H
1664:3
1662:C
1657:8
1655:H
1653:3
1651:C
1647:2
1643:2
1639:3
1635:3
1631:4
1625:3
1621:3
1616:)
1614:2
1610:3
1606:2
1602:2
1597:3
1593:3
1591:H
1587:3
1582:4
1562:3
1558:2
1550:O
1548:2
1544:2
1534:2
1530:2
1528:H
1497:2
1485:2
1370:2
1344:4
1328:2
1316:2
1308:2
1304:0
1300:2
1296:2
1292:4
1288:4
1286:E
1270:0
1261:4
1259:E
1252:3
1250:E
1244:2
1235:2
1233:E
1215:2
1210:1
1208:E
1202:2
1193:0
1191:E
1181:4
1177:0
1169:2
1165:3
1161:2
1157:n
1136:3
1132:2
1118:)
1116:2
1114:(
1107:i
1103:2
1099:4
1095:2
1093:N
1083:)
1081:1
1079:(
1072:i
1068:2
1064:3
1060:2
1058:N
1034:3
1030:2
950:2
934:9
930:7
923:7
921:S
919:8
825:3
821:2
774:1
767:l
763:o
760:m
754:J
751:k
735:=
729:A
724:E
696:3
692:H
688:N
680:1
673:l
669:o
666:m
660:J
657:k
644:=
639:0
635:H
623:(
591:(
577:3
569:2
534:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.