466:
289:
517:
493:
422:
231:
148:
375:
257:
485:, a natural compound found in common foods like grapes, wines and nuts. Resveratrol is a biologically important stilbenoid which has been suggested to have many health benefits. The Julia-Kocienski olefination serves as a powerful reaction in the synthesis of resveratrol analogues with 3,5-bis(trifluoromethyl)phenyl sulfones. The following schematic displays the general scheme for synthesizing resveratrol analogues, where R
196:
reactions, the elimination was done under reductive conditions. More recently, a modified version that avoids this step was developed. The former version is sometimes referred to as the Julia-Lythgoe olefination, whereas the latter is called the Julia-Kocienski olefination. In the reductive variant, the adduct is usually acylated and then treated with a reducing agent, such as
238:
The stereochemistry of the alkene (6) is independent of the stereochemistry of the sulfone intermediate 4. It is thought that the radical intermediates are able to equilibrate so that the more thermodynamically stable trans-olefin is produced most often. This transformation highly favors formation of
195:
explored the scope and limitation of the reaction, and today this olefination is formally known as the Julia-Lythgoe olefination. The reaction involves the addition of a sulfonyl-stabilized carbanion to a carbonyl compound, followed by elimination to form an alkene. In the initial versions of the
296:
Since the benzothiazole variation of the Julia olefination does not involve equilibrating intermediates, the stereochemical outcome is a result of the stereochemistry of the initial carbonyl addition. As a result, this reaction often generates a mixture of alkene stereoisomers.
398:-selectivity of the Julia–Kocienski olefination is the result of kinetically controlled diastereoselective addition of metalated 1-phenyl-1H-tetrazol-5-yl (PT) sulfones to nonconjugated aldehydes. This yields anti-β-alkoxysulfones which stereospecifically decompose to the
264:
The modified Julia olefination, also known as the one-pot Julia olefination is a modification of the classical Julia olefination. The replacement of the phenyl sulfones with heteroaryl sulfones greatly alters the reaction pathway. The most popular example is the
446:
is a stilbenoid chemically related to resveratrol. It belongs to the group of phytoalexins, agents produced by plants to fight infections. Pterostilbene is a naturally occurring dimethyl ether analog of resveratrol. It is believed that the compound also has
513:-alkene moieties. (−)-callystatin A is a member of the leptomycin family of antibiotics. The following schematic displays the Julia-Kocienski olefination used to achieve the precursor to the natural product, as indicated by use of the PT-sulfone.
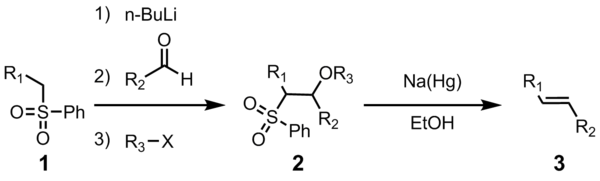
227:-X to give the stable intermediate (4). The exact mechanism of the sodium amalgam reduction is unknown but has been shown to proceed through a vinylic radical species (5). Protonation of the vinylic radical gives the desired product (6).
273:(LDA) gives a metallated benzothiazolyl sulfone, which reacts quickly with aldehydes (or ketones) to give an alkoxide intermediate (2). Unlike the phenyl sulfones, this alkoxide intermediate (2) is more reactive and will undergo a
434:
The Julia or modified Julia olefination reaction is a powerful and versatile synthetic transformation, widely utilized in the construction of complex natural products with excellent control of geometrical isomerism.
190:
In 1973, Marc Julia and Jean-Marc Paris reported a novel olefin synthesis in which β-acyloxysulfones were reductively eliminated to the corresponding di-, tri-, or tetrasubstituted alkenes. Basil
Lythgoe and
817:
Moro, A. V.; Cardoso, F. S. P.; Correia, C. R. D. Heck arylation of styrenes with arenediazonium salts: Short, efficient, and stereoselective synthesis of resveratrol, DMU-212, and analogues.
155:
The utility of this connective olefination reaction arises from its versatility, its wide functional group tolerance, and the mild reaction conditions under which the reaction proceeds.
462:, or transition-metal-catalyzed reactions to synthesize pterostilebene, the Julia olefination offers a simple, economical alternative method for preparation of pterostilbene.
288:
465:
828:
Prabhakar
Peddikotla, Amar G. Chittiboyina, Ikhlas A. Khan, (2014) ChemInform Abstract: Synthesis of Pterostilbene by Julia Olefination. ChemInform 45,
17:
1365:
361:
81:
535:
1178:
873:
Robiette, R.; Pospíšil, J. On the Origin of E/Z Selectivity in the
Modified Julia Olefination: Importance of the Elimination Step;
530:
516:
492:
897:
346:
421:
230:
147:
374:
256:
1163:
924:
1208:
1375:
407:
162:
X is optional. However, purification of the sulfone intermediate 2 leads to higher yield and purity. Most often R
418:
at +70 °C. This reaction is named after Philip J. Kocienski for his modification to the Julia olefination.
1360:
664:
588:
1138:
892:
382:
The Julia–Kocienski
Olefination, a further refinement of the Modified Julia olefination, offers very good
66:
1370:
1355:
1143:
354:
74:
1334:
1314:
270:
1380:
1274:
320:
1269:
1148:
771:
Zajc, B., & Kumar, R. (2010). Synthesis of
Fluoroolefins via Julia-Kocienski Olefination.
1193:
917:
1203:
1183:
1120:
540:
274:
8:
1173:
1153:
1124:
1111:
772:
201:
134:
1264:
1238:
1168:
874:
652:
698:
576:
1304:
1228:
1218:
1129:
848:
818:
803:
685:
563:
415:
330:
107:
103:
50:
277:
to give the sulfinate salt (4). The sulfinate salt (4) will spontaneously eliminate
1284:
1213:
1198:
910:
829:
795:
776:
757:
732:
694:
669:
648:
610:
572:
394:. It proceeds with the same mechanism as the benzothiazole sulfone above. The high
192:
179:
175:
790:
Langcake, P.; Pryce, R. J. (1977). "A new class of phytoalexins from grapevines".
1324:
1294:
1223:
545:
459:
455:
411:
1319:
1309:
278:
197:
130:
1349:
1279:
1254:
1158:
844:
748:
601:
478:
One adaptation of the Julia-Kocienski olefination gives the synthesis of the
448:
443:
266:
129:(olefins)(3) after alcohol functionalization and reductive elimination using
1329:
1259:
1233:
852:
833:
780:
158:
All four steps can be carried out in a single reaction vessel, and use of R
1289:
887:
862:
A. B. Smith, III and B. M. Brandt. Total
Synthesis of (–)-Callystatin A.
807:
791:
710:
482:
736:
614:
1299:
1115:
863:
799:
725:
Paul R. Blakemore, William J. Cole, Philip J. Kocieński, Andrew Morley
506:
479:
387:
316:
141:
40:
761:
673:
391:
292:
The mechanism of the benzothiazole variation of the Julia olefination
118:
947:
451:
properties, but so far very little has been studied on this issue.
220:
1032:
998:
964:
727:
282:
171:
114:
1133:
1083:
1066:
1049:
1015:
981:
933:
167:
126:
122:
111:
269:
sulfone. The reaction of the benzothiazole sulfone (1) with
216:
902:
509:, two separate Julia olefinations were used to append two
505:
In the asymmetric total synthesis of (−)-callystatin A by
215:
The initial steps are straightforward. The phenyl sulfone
207:. Several reviews of these reactions have been published.
285:
benzothiazolone (5) producing the desired alkene (6).
469:
386:-selectivity. In the Julia–Kocienski olefination the
683:Baudin, J. B.; Hareau, G.; Julia, S. A.; Ruel, O.
140:. The reaction is named after the French chemist
1347:
300:
918:
251:
843:Alonso DA, Fuensanta M, Nájera C, Varea M.
708:Truce, W. E.; Kreider, E. M.; Brand, W. W.
925:
911:
658:
620:
599:Keck, G. E.; Savin, K. A.; Weglarz, M. A.
586:Kocienski, P. J.; Lythgoe, B.; Ruston, S.
582:
223:(3). The alkoxide is functionalized with R
429:
219:(2) reacts with an aldehyde to form the
824:
679:
14:
1348:
721:
704:
635:
557:
906:
869:
786:
595:
210:
1366:Carbon-carbon bond forming reactions
500:
347:modified-julia-kocienski-olefination
839:
520:Julia olefination for callystatin A
496:General Resveratrol Analogue Scheme
406:the reaction conditions are either
24:
858:
742:
653:10.1016/B978-0-08-052349-1.00020-2
515:
491:
464:
420:
402:-alkenes. In one adaptation, with
373:
287:
255:
229:
146:
25:
1392:
881:
813:
767:
536:Johnson–Corey–Chaykovsky reaction
1179:Horner–Wadsworth–Emmons reaction
531:Horner–Wadsworth–Emmons reaction
438:
425:Julia-Kocienski olefination wiki
234:Julia olefination mechanism wiki
151:Julia Olefination Revised Scheme
641:Comprehensive Organic Synthesis
408:sodium bis(trimethylsilyl)amide
665:J. Chem. Soc., Perkin Trans. 1
589:J. Chem. Soc., Perkin Trans. 1
473:
404:t-butyltetrazoylmethyl sulfone
378:General julia kocienski scheme
182:used in the preparation of 2.
13:
1:
1164:Corey–Winter olefin synthesis
699:10.1016/S0040-4039(00)92037-9
577:10.1016/S0040-4039(01)87348-2
551:
260:General modified julia scheme
246:
308:Julia–Kocienski olefination
7:
932:
898:Julia-Kocienski Olefination
524:
301:Julia–Kocienski olefination
18:Julia–Kocienski olefination
10:
1397:
252:Modified Julia olefination
185:
1335:Friedel-Crafts Alkylation
1247:
1209:Ramberg–Bäcklund reaction
1104:
940:
888:Julia-Lythgoe Olefination
368:
342:Organic Chemistry Portal
336:
307:
88:
62:Organic Chemistry Portal
56:
31:
1139:Bamford–Stevens reaction
821:2008, 49(39), 5668–5671.
561:Julia, M.; Paris, J.-M.
271:lithium diisopropylamide
1275:Oxymercuration reaction
1144:Barton–Kellogg reaction
775:, 2010(11), 1822–1836.(
321:Philip Joseph Kocienski
1376:Free radical reactions
1270:Electrophilic addition
1149:Boord olefin synthesis
834:10.1002/chin.201408101
781:10.1055/s-0029-1218789
521:
497:
470:
430:Synthetic Applications
426:
379:
293:
261:
235:
152:
1361:Olefination reactions
1194:Kauffmann olefination
647:, 792–806. (Review) (
626:Phosphorus and Sulfur
519:
495:
468:
424:
377:
291:
259:
233:
150:
1315:Diels–Alder reaction
1204:Peterson olefination
1184:Hydrazone iodination
1121:Dehydration reaction
847:2005; 70:6404–6416.
541:Peterson olefination
275:Smiles rearrangement
102:olefination) is the
1174:Hofmann elimination
1154:Chugaev elimination
1112:Dehydrohalogenation
866:2001, 3, 1685–1688.
737:10.1055/s-1998-1570
615:10.1021/jo00115a041
193:Philip J. Kocienski
98:(also known as the
1371:Addition reactions
1356:Coupling reactions
1239:Cope rearrangement
1169:Grieco elimination
875:Eur. J. Org. Chem.
800:10.1007/BF02124034
668:2002, 2563–2585. (
632:, 97–127. (Review)
522:
498:
489:is an aryl group.
471:
427:
410:at −70 °C in
380:
294:
262:
236:
211:Reaction mechanism
153:
32:Julia olefination
1343:
1342:
1305:Hydrohalogenation
1229:Olefin metathesis
1219:Takai olefination
1189:Julia olefination
1130:Semihydrogenation
893:Julia Olefination
819:Tetrahedron Lett.
762:10.1021/jo051693a
746:Christophe Aïssa
686:Tetrahedron Lett.
662:Blakemore, P. R.
624:Kocienski, P. J.
564:Tetrahedron Lett.
501:(−)-Callystatin A
458:, Wittig-Horner,
416:caesium carbonate
372:
371:
331:Coupling reaction
108:organic chemistry
104:chemical reaction
96:Julia olefination
92:
91:
67:julia-olefination
51:Coupling reaction
16:(Redirected from
1388:
1285:Cyclopropanation
1214:Shapiro reaction
1199:McMurry reaction
1096:
1079:
1062:
1045:
1028:
1011:
994:
977:
960:
927:
920:
913:
904:
903:
872:
861:
842:
827:
816:
794:33 (2): 151–2. (
789:
770:
745:
724:
707:
682:
674:10.1039/b208078h
661:
638:
623:
598:
585:
560:
454:Compared to the
388:alkylating agent
364:
349:
305:
304:
180:benzoyl chloride
176:acetic anhydride
84:
69:
29:
28:
21:
1396:
1395:
1391:
1390:
1389:
1387:
1386:
1385:
1346:
1345:
1344:
1339:
1325:Dehydrogenation
1295:Dihydroxylation
1243:
1224:Wittig reaction
1100:
1095:
1091:
1087:
1078:
1074:
1070:
1061:
1057:
1053:
1044:
1040:
1036:
1027:
1023:
1019:
1010:
1006:
1002:
993:
989:
985:
976:
972:
968:
959:
955:
951:
936:
931:
884:
554:
546:Wittig reaction
527:
503:
488:
476:
441:
432:
412:tetrahydrofuran
360:
345:
319:
303:
254:
249:
226:
213:
205:
188:
165:
161:
138:
80:
65:
23:
22:
15:
12:
11:
5:
1394:
1384:
1383:
1381:Name reactions
1378:
1373:
1368:
1363:
1358:
1341:
1340:
1338:
1337:
1332:
1327:
1322:
1320:Wacker process
1317:
1312:
1310:Polymerization
1307:
1302:
1297:
1292:
1287:
1282:
1277:
1272:
1267:
1262:
1257:
1251:
1249:
1245:
1244:
1242:
1241:
1236:
1231:
1226:
1221:
1216:
1211:
1206:
1201:
1196:
1191:
1186:
1181:
1176:
1171:
1166:
1161:
1156:
1151:
1146:
1141:
1136:
1127:
1118:
1108:
1106:
1102:
1101:
1099:
1098:
1093:
1089:
1081:
1076:
1072:
1064:
1059:
1055:
1047:
1042:
1038:
1030:
1025:
1021:
1013:
1008:
1004:
996:
991:
987:
979:
974:
970:
962:
957:
953:
944:
942:
938:
937:
930:
929:
922:
915:
907:
901:
900:
895:
890:
883:
882:External links
880:
879:
878:
877:2013, 836–840.
867:
856:
837:
822:
811:
784:
765:
740:
731:1998, 26–28. (
719:
718:, 99. (Review)
702:
677:
656:
633:
618:
609:, 3194–3204. (
593:
580:
571:, 4833–4836. (
553:
550:
549:
548:
543:
538:
533:
526:
523:
502:
499:
486:
475:
472:
440:
437:
431:
428:
370:
369:
366:
365:
358:
351:
350:
343:
339:
338:
334:
333:
328:
327:Reaction type
324:
323:
314:
310:
309:
302:
299:
279:sulfur dioxide
253:
250:
248:
245:
224:
212:
209:
203:
198:sodium amalgam
187:
184:
163:
159:
136:
131:sodium amalgam
90:
89:
86:
85:
78:
71:
70:
63:
59:
58:
54:
53:
48:
47:Reaction type
44:
43:
38:
34:
33:
9:
6:
4:
3:
2:
1393:
1382:
1379:
1377:
1374:
1372:
1369:
1367:
1364:
1362:
1359:
1357:
1354:
1353:
1351:
1336:
1333:
1331:
1328:
1326:
1323:
1321:
1318:
1316:
1313:
1311:
1308:
1306:
1303:
1301:
1298:
1296:
1293:
1291:
1288:
1286:
1283:
1281:
1280:Hydroboration
1278:
1276:
1273:
1271:
1268:
1266:
1263:
1261:
1258:
1256:
1255:Hydrogenation
1253:
1252:
1250:
1246:
1240:
1237:
1235:
1232:
1230:
1227:
1225:
1222:
1220:
1217:
1215:
1212:
1210:
1207:
1205:
1202:
1200:
1197:
1195:
1192:
1190:
1187:
1185:
1182:
1180:
1177:
1175:
1172:
1170:
1167:
1165:
1162:
1160:
1159:Cope reaction
1157:
1155:
1152:
1150:
1147:
1145:
1142:
1140:
1137:
1135:
1131:
1128:
1126:
1122:
1119:
1117:
1113:
1110:
1109:
1107:
1103:
1085:
1082:
1068:
1065:
1051:
1048:
1034:
1031:
1017:
1014:
1000:
997:
983:
980:
966:
963:
949:
946:
945:
943:
939:
935:
928:
923:
921:
916:
914:
909:
908:
905:
899:
896:
894:
891:
889:
886:
885:
876:
871:
868:
865:
860:
857:
854:
850:
846:
845:J. Org. Chem.
841:
838:
835:
831:
826:
823:
820:
815:
812:
809:
805:
801:
797:
793:
788:
785:
782:
778:
774:
769:
766:
763:
759:
755:
751:
750:
749:J. Org. Chem.
744:
741:
738:
734:
730:
729:
723:
720:
717:
713:
712:
706:
703:
700:
696:
692:
688:
687:
681:
678:
675:
671:
667:
666:
660:
657:
654:
650:
646:
642:
639:Kelly, S. E.
637:
634:
631:
627:
622:
619:
616:
612:
608:
604:
603:
602:J. Org. Chem.
597:
594:
591:
590:
584:
581:
578:
574:
570:
566:
565:
559:
556:
555:
547:
544:
542:
539:
537:
534:
532:
529:
528:
518:
514:
512:
508:
494:
490:
484:
481:
467:
463:
461:
457:
452:
450:
449:anti-diabetic
445:
444:Pterostilbene
439:Pterostilbene
436:
423:
419:
417:
413:
409:
405:
401:
397:
393:
389:
385:
376:
367:
363:
359:
356:
353:
352:
348:
344:
341:
340:
335:
332:
329:
326:
325:
322:
318:
315:
312:
311:
306:
298:
290:
286:
284:
280:
276:
272:
268:
267:benzothiazole
258:
244:
242:
232:
228:
222:
218:
208:
206:
199:
194:
183:
181:
177:
173:
169:
156:
149:
145:
143:
139:
132:
128:
124:
120:
116:
113:
109:
105:
101:
100:Julia–Lythgoe
97:
87:
83:
79:
76:
73:
72:
68:
64:
61:
60:
55:
52:
49:
46:
45:
42:
39:
36:
35:
30:
27:
19:
1330:Ene reaction
1260:Halogenation
1234:Ene reaction
1188:
1105:Preparations
870:
859:
840:
825:
814:
787:
768:
753:
747:
743:
726:
722:
715:
709:
705:
690:
684:
680:
663:
659:
644:
640:
636:
629:
625:
621:
606:
600:
596:
587:
583:
568:
562:
558:
510:
504:
477:
453:
442:
433:
403:
399:
395:
383:
381:
362:RXNO:0000304
357:ontology ID
337:Identifiers
313:Named after
295:
263:
240:
237:
214:
189:
157:
154:
99:
95:
93:
82:RXNO:0000117
77:ontology ID
57:Identifiers
37:Named after
26:
1290:Epoxidation
792:Experientia
756:, 360–63. (
711:Org. React.
483:resveratrol
474:Resveratrol
1350:Categories
1300:Ozonolysis
1116:haloalkane
864:Org. Lett.
592:1978, 829.
552:References
507:Amos Smith
480:stilbenoid
317:Marc Julia
247:Variations
142:Marc Julia
125:) to give
41:Marc Julia
1265:Hydration
1248:Reactions
773:Synthesis
693:, 1175. (
392:tetrazole
243:-alkene.
119:aldehydes
117:(1) with
853:16050703
525:See also
221:alkoxide
115:sulfones
106:used in
1125:alcohol
1033:Heptene
999:Pentene
965:Propene
941:Alkenes
934:Alkenes
728:Synlett
283:lithium
186:History
174:, with
172:benzoyl
127:alkenes
123:ketones
1134:alkyne
1084:Decene
1067:Nonene
1050:Octene
1016:Hexene
982:Butene
948:Ethene
851:
808:844529
806:
752:2006,
714:1970,
689:1991,
643:1991,
628:1985,
605:1995,
567:1973,
460:Perkin
456:Wittig
168:acetyl
112:phenyl
1132:from
1123:from
1114:from
390:is a
217:anion
849:PMID
804:PMID
281:and
239:the
121:(or
94:The
830:doi
796:doi
777:doi
758:doi
733:doi
695:doi
670:doi
649:doi
611:doi
573:doi
414:or
355:RSC
202:SmI
200:or
178:or
170:or
166:is
135:SmI
133:or
110:of
75:RSC
1352::
1094:20
1090:10
1077:18
1060:16
1043:14
1026:12
1009:10
802:)
754:71
716:18
691:32
630:24
607:60
569:14
144:.
1097:)
1092:H
1088:C
1086:(
1080:)
1075:H
1073:9
1071:C
1069:(
1063:)
1058:H
1056:8
1054:C
1052:(
1046:)
1041:H
1039:7
1037:C
1035:(
1029:)
1024:H
1022:6
1020:C
1018:(
1012:)
1007:H
1005:5
1003:C
1001:(
995:)
992:8
990:H
988:4
986:C
984:(
978:)
975:6
973:H
971:3
969:C
967:(
961:)
958:4
956:H
954:2
952:C
950:(
926:e
919:t
912:v
855:.
836:.
832::
810:.
798::
783:)
779::
764:)
760::
739:)
735::
701:)
697::
676:)
672::
655:)
651::
645:1
617:)
613::
579:)
575::
511:E
487:2
400:E
396:E
384:E
241:E
225:3
204:2
164:3
160:3
137:2
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.