610:
630:
122:
25:
600:
wavelengths as concentrations change, creating the impression that the isosbestic point is 'out of focus', or that it will shift as conditions change. The reason for this is that it would be very unlikely for three compounds to have extinction coefficients linked in a linear relationship by chance
589:
The requirement for an isosbestic point to occur is that the two species involved are related linearly by stoichiometry, such that the absorbance is invariant at a certain wavelength. Thus, ratios other than 1-to-1 are possible. The presence of an isosbestic point typically indicates that only
145:
is a specific wavelength, wavenumber or frequency at which the total absorbance of a sample does not change during a chemical reaction or a physical change of the sample. The word derives from two Greek words: "iso", meaning "equal", and "sbestos", meaning "extinguishable".
581:
380:
434:
446:
295:
242:
596:
species that vary in concentration contribute to the absorption around the isosbestic point. If a third one is partaking in the process, the spectra typically intersect at
879:
200:) involves a pair of substances with an isosbestic point, the absorbance of the reaction mixture at this wavelength remains invariant, regardless of the
817:
307:
89:
863:
392:
61:
576:{\displaystyle A=l\cdot (\epsilon _{X}c_{X}+\epsilon _{Y}c_{Y})=l\cdot \epsilon \cdot (c_{X}+c_{Y})=l\cdot \epsilon \cdot c}
795:
652:
68:
855:
108:
42:
780:
75:
46:
940:: Total synthesis of vitamin B12 provided a framework for exploration in several areas of organic chemistry".
57:
609:
787:. The isosbestic points provide proof for a direct conversion of the seco-corrin complex to the metal-free
253:
221:
209:
130:
35:
586:
does not depend on the extent of reaction (i.e., on the particular concentrations of X and Y)
82:
991:
951:
739:
641:
197:
629:
8:
189:
955:
750:(541 nm). The wavelength of the isosbestic point determined does not depend on the
933:
773:
769:
697:
637:
201:
159:
204:(or the position of the chemical equilibrium). This occurs because the two substances
967:
942:
859:
851:
758:
721:
713:
701:
659:
193:
126:
827:
959:
915:
905:
831:
822:
743:
686:
663:
129:
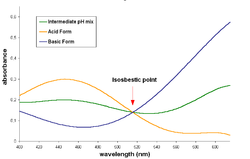
spectrum. The spectra of basic, acid and intermediate pH solutions are shown. The
301:
The absorbance of the reaction mixture (assuming it depends only on X and Y) is:
919:
762:
682:
634:
622:
618:
985:
910:
847:
826:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
751:
121:
901:
835:
777:
963:
717:
155:
138:
173:
A pair of substances can have several isosbestic points in their spectra.
971:
614:
185:
177:
673:
Isosbestic points are used in medicine in a laboratory technique called
709:
678:
667:
247:
the analytical concentration is the same at any point in the reaction:
205:
163:
794:
without intermediary or side products (within the detection limits of
747:
690:
386:
But at the isosbestic point, both molar absorptivities are the same:
170:
corresponds to a wavelength at which these spectra cross each other.
754:
of the substance used, and so it becomes a very reliable reference.
24:
705:
674:
181:
154:
When an isosbestic plot is constructed by the superposition of the
850:
and Arthur
Atwater Frost (3rd Edition, John Wiley and Sons, 1981)
936:; Wintner, C. E. (1977). "Natural Product Synthesis and Vitamin B
662:, isosbestic points are used as reference points in the study of
375:{\displaystyle A=l\cdot (\epsilon _{X}c_{X}+\epsilon _{Y}c_{Y})}
166:
and keeping the same molar concentration for both species), the
791:
788:
766:
648:
645:
208:
light of that specific wavelength to the same extent, and the
729:
725:
429:{\displaystyle \epsilon _{X}=\epsilon _{Y}=\epsilon \,}
644:
of an A/D-seco-corrin to the corresponding metal-free
772:
ring closure reaction, which was the key step in the
689:
have (not exclusively) isosbestic points at 586
449:
395:
310:
256:
224:
846:
page 49 of
Kinetics and Mechanism By John W. Moore,
49:. Unsourced material may be challenged and removed.
932:
575:
428:
374:
289:
236:
983:
670:remains constant throughout the whole reaction.
757:One example of the use of isosbestic points in
738:of the substance). The standards used include
681:concentration, regardless of its saturation.
742:(isosbestic points at 339 and 445 nm),
625:, shows an isosbestic point near 808 nm
16:Spectroscopic property in chemical reactions
873:
871:
813:
811:
909:
425:
286:
109:Learn how and when to remove this message
868:
628:
608:
133:of the dye is the same in all solutions.
120:
808:
984:
877:
162:for the representation, or by using
47:adding citations to reliable sources
18:
696:Isosbestic points are also used in
13:
823:Compendium of Chemical Terminology
149:
14:
1003:
158:of two species (whether by using
728:conditions (above and below the
716:. This is done by measuring the
23:
604:
601:for one particular wavelength.
290:{\displaystyle c_{X}+c_{Y}=c\,}
34:needs additional citations for
926:
840:
552:
526:
508:
462:
369:
323:
237:{\displaystyle X\rightarrow Y}
228:
1:
904:(Promotionsarbeit Nr. 5158).
801:
666:, as the absorbance at those
890:Total Synthesis of Vitamin B
7:
881:Totalsynthese von Vitamin B
10:
1008:
746:(325 and 498 nm) and
894:: The Photochemical Route
621:system, measured through
911:10.3929/ethz-a-000086601
885:: Der photochemische Weg
210:analytical concentration
131:analytical concentration
125:Isosbestic point in the
878:Fuhrer, Walter (1973).
836:10.1351/goldbook.I03310
440:Hence, the absorbance
964:10.1126/science.867037
704:method, to verify the
693:and near 808 nm.
655:
626:
577:
430:
376:
291:
238:
134:
632:
612:
578:
431:
377:
292:
239:
124:
740:potassium dichromate
447:
393:
308:
254:
222:
43:improve this article
956:1977Sci...196.1410E
950:(4297): 1410–1420.
920:20.500.11850/131362
900:(PhD) (in German).
796:UV/VIS spectroscopy
653:UV/VIS spectroscopy
176:When a 1-to-1 (one
770:cycloisomerization
698:clinical chemistry
656:
638:cycloisomerization
627:
573:
426:
372:
287:
234:
215:For the reaction:
212:remains constant.
202:extent of reaction
160:molar absorptivity
156:absorption spectra
135:
58:"Isosbestic point"
864:978-0-471-03558-9
759:organic synthesis
724:at two different
722:standard solution
714:spectrophotometer
702:quality assurance
660:chemical kinetics
194:chemical reaction
127:bromocresol green
119:
118:
111:
93:
999:
976:
975:
930:
924:
923:
913:
899:
875:
866:
848:Ralph G. Pearson
844:
838:
828:isosbestic point
815:
744:bromothymol blue
687:deoxyhaemoglobin
582:
580:
579:
574:
551:
550:
538:
537:
507:
506:
497:
496:
484:
483:
474:
473:
435:
433:
432:
427:
418:
417:
405:
404:
381:
379:
378:
373:
368:
367:
358:
357:
345:
344:
335:
334:
296:
294:
293:
288:
279:
278:
266:
265:
243:
241:
240:
235:
168:isosbestic point
143:isosbestic point
114:
107:
103:
100:
94:
92:
51:
27:
19:
1007:
1006:
1002:
1001:
1000:
998:
997:
996:
982:
981:
980:
979:
939:
934:Eschenmoser, A.
931:
927:
897:
893:
884:
876:
869:
845:
841:
816:
809:
804:
785:total synthesis
784:
761:is seen in the
736:
607:
546:
542:
533:
529:
502:
498:
492:
488:
479:
475:
469:
465:
448:
445:
444:
413:
409:
400:
396:
394:
391:
390:
363:
359:
353:
349:
340:
336:
330:
326:
309:
306:
305:
274:
270:
261:
257:
255:
252:
251:
223:
220:
219:
152:
150:Isosbestic plot
115:
104:
98:
95:
52:
50:
40:
28:
17:
12:
11:
5:
1005:
995:
994:
978:
977:
937:
925:
891:
882:
867:
839:
806:
805:
803:
800:
782:
734:
683:Oxyhaemoglobin
664:reaction rates
633:Following the
623:pulse oximetry
619:oxyhaemoglobin
606:
603:
584:
583:
572:
569:
566:
563:
560:
557:
554:
549:
545:
541:
536:
532:
528:
525:
522:
519:
516:
513:
510:
505:
501:
495:
491:
487:
482:
478:
472:
468:
464:
461:
458:
455:
452:
438:
437:
424:
421:
416:
412:
408:
403:
399:
384:
383:
371:
366:
362:
356:
352:
348:
343:
339:
333:
329:
325:
322:
319:
316:
313:
299:
298:
285:
282:
277:
273:
269:
264:
260:
245:
244:
233:
230:
227:
151:
148:
117:
116:
31:
29:
22:
15:
9:
6:
4:
3:
2:
1004:
993:
990:
989:
987:
973:
969:
965:
961:
957:
953:
949:
945:
944:
935:
929:
921:
917:
912:
907:
903:
895:
887:
886:
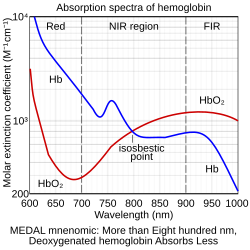
874:
872:
865:
861:
857:
856:0-471-03558-0
853:
849:
843:
837:
833:
829:
825:
824:
819:
814:
812:
807:
799:
797:
793:
790:
786:
779:
775:
771:
768:
764:
763:photochemical
760:
755:
753:
752:concentration
749:
745:
741:
737:
733:
727:
723:
719:
715:
711:
707:
703:
699:
694:
692:
688:
684:
680:
677:to determine
676:
671:
669:
665:
661:
654:
650:
647:
643:
639:
636:
635:photochemical
631:
624:
620:
616:
611:
602:
599:
595:
594:
587:
570:
567:
564:
561:
558:
555:
547:
543:
539:
534:
530:
523:
520:
517:
514:
511:
503:
499:
493:
489:
485:
480:
476:
470:
466:
459:
456:
453:
450:
443:
442:
441:
422:
419:
414:
410:
406:
401:
397:
389:
388:
387:
364:
360:
354:
350:
346:
341:
337:
331:
327:
320:
317:
314:
311:
304:
303:
302:
283:
280:
275:
271:
267:
262:
258:
250:
249:
248:
231:
225:
218:
217:
216:
213:
211:
207:
203:
199:
195:
191:
187:
183:
179:
174:
171:
169:
165:
161:
157:
147:
144:
140:
132:
128:
123:
113:
110:
102:
91:
88:
84:
81:
77:
74:
70:
67:
63:
60: –
59:
55:
54:Find sources:
48:
44:
38:
37:
32:This article
30:
26:
21:
20:
992:Spectroscopy
947:
941:
928:
889:
880:
842:
821:
756:
731:
695:
672:
657:
605:Applications
597:
592:
591:
588:
585:
439:
385:
300:
246:
214:
175:
172:
167:
153:
142:
139:spectroscopy
136:
105:
96:
86:
79:
72:
65:
53:
41:Please help
36:verification
33:
774:Eschenmoser
668:wavelengths
615:haemoglobin
196:(including
902:ETH Zürich
802:References
778:ETH Zürich
710:wavelength
679:hemoglobin
198:equilibria
184:gives one
164:absorbance
99:March 2022
69:newspapers
781:vitamin B
748:congo red
568:⋅
565:ϵ
562:⋅
524:⋅
521:ϵ
518:⋅
490:ϵ
467:ϵ
460:⋅
423:ϵ
411:ϵ
398:ϵ
351:ϵ
328:ϵ
321:⋅
229:→
986:Category
706:accuracy
675:oximetry
640:of a Cd
182:reactant
952:Bibcode
943:Science
718:spectra
708:in the
700:, as a
642:complex
598:varying
190:product
83:scholar
972:867037
970:
896:]
862:
854:
792:ligand
789:corrin
767:corrin
649:ligand
646:corrin
206:absorb
85:
78:
71:
64:
56:
898:(PDF)
888:[
818:IUPAC
720:of a
712:of a
141:, an
90:JSTOR
76:books
968:PMID
860:ISBN
852:ISBN
798:).
765:A/D-
685:and
613:The
186:mole
178:mole
62:news
960:doi
948:196
916:hdl
906:doi
832:doi
830:".
658:In
651:by
593:two
188:of
180:of
137:In
45:by
988::
966:.
958:.
946:.
938:12
914:.
892:12
883:12
870:^
858:,
820:,
810:^
783:12
776:/
726:pH
691:nm
617:/
192:)
974:.
962::
954::
922:.
918::
908::
834::
735:a
732:K
730:p
571:c
559:l
556:=
553:)
548:Y
544:c
540:+
535:X
531:c
527:(
515:l
512:=
509:)
504:Y
500:c
494:Y
486:+
481:X
477:c
471:X
463:(
457:l
454:=
451:A
436:.
420:=
415:Y
407:=
402:X
382:.
370:)
365:Y
361:c
355:Y
347:+
342:X
338:c
332:X
324:(
318:l
315:=
312:A
297:.
284:c
281:=
276:Y
272:c
268:+
263:X
259:c
232:Y
226:X
112:)
106:(
101:)
97:(
87:·
80:·
73:·
66:·
39:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.