125:
200:
260:
of nanometers, comparable to or smaller than the lamellar crystal thickness or the radius of gyration, nucleation and growth can be dramatically affected. As an example, when a polymer crystallizes in a confined ultrathin layer, the isotropic spherulitic organization of lamellar crystals is hampered and confinement can produce unique lamellar crystal orientations. Sometimes the chain alignment is parallel to the layer plane and the crystals are organized as ‘‘on-edge’’ lamellae. In other cases, "in-plane" lamellae with chain orientation perpendicular to the layers are observed.
48:
243:
occur via solvent evaporation, induces interaction between molecular chains and a possible crystallization as in the crystallization from the melt. Crystallization from solution may result in the highest degree of polymer crystallinity. For example, highly linear polyethylene can form platelet-like single crystals with a thickness on the order 10–20 nm when crystallized from a dilute solution. The crystal shape can be more complex for other polymers, including hollow pyramids, spirals and multilayer dendritic structures.
629:
stress or shearing. Evidence suggests that cavitation also impacts the onset of yielding. The voids are associated with the breaking of the amorphous phase. The strength of the crystalline phase determines the importance of cavitation in yielding. If the crystalline structures are weak, they deform easily resulting in yielding. Semi-crystalline polymers with strong crystalline regions resist deformation and cavitation, the formation of voids in the amorphous phase, drives yielding.
84:
92:
61:
typical size of the order 1 micrometer. Although it would be energetically favorable for the polymer chains to align parallel, such alignment is hindered by the entanglement. Therefore, within the ordered regions, the polymer chains are both aligned and folded. Those regions are therefore neither crystalline nor amorphous and are classified as semicrystalline. Examples of semi-crystalline polymers are linear
191:
quasi-spherical aggregates called spherulites. Spherulites have a size between about 1 and 100 micrometers and form a large variety of colored patterns (see, e.g. front images) when observed between crossed polarizers in an optical microscope, which often include the "maltese cross" pattern and other polarization phenomena caused by molecular alignment within the individual lamellae of a spherulite.
149:
279:
polymerization happens in the crystalline lattice without the aid of solvents or reagents, it comes under the domain of green chemistry. Also, the topochemical polymerizations are mostly atom economical reactions. The product can be obtained without any further purifications. It can achieve unique products which cannot be synthesized through conventional methods.
617:
load. When a tensile stress is applied the semi-crystalline polymer first deforms elastically. While the crystalline regions remain unaffected by the applied stress, the molecular chains of the amorphous phase stretch. Then yielding, which signifies the onset of plastic deformation of the crystalline regions, occurs.
613:
and more thermally stable, but also more brittle material, whereas the amorphous regions provide certain elasticity and impact resistance. Another characteristic feature of semicrystalline polymers is strong anisotropy of their mechanical properties along the direction of molecular alignment and perpendicular to it.
625:
significantly following neck propagation. Mechanical anisotropy increases and the elastic modulus varies along different directions, with a high modulus observed in the draw direction. Drawn semi-crystalline polymers are the strongest polymeric materials due to the stress-induced ordering of the molecular chains.
596:
Below their glass transition temperature, amorphous polymers are usually hard and brittle because of the low mobility of their molecules. Increasing the temperature induces molecular motion resulting in the typical rubber-elastic properties. A constant force applied to a polymer at temperatures above
553:
Infrared absorption or reflection spectra from crystalline polymers contain additional peaks which are absent in amorphous materials with the same composition. These signals may originate from deformation vibrations of the regular arrangement of molecular chains. From the analysis of these bands, the
620:
The molecular mechanism for semi-crystalline yielding involves the deformation of crystalline regions of the material via dislocation motion. Dislocations result in coarse or fine slips in the polymer and lead to crystalline fragmentation and yielding. Fine slip is defined as a small amount of slip
612:
Relatively strong intermolecular forces in semicrystalline polymers prevent softening even above the glass transition temperature. Their elastic modulus changes significantly only at high (melting) temperature. It also depends on the degree of crystallinity: higher crystallinity results in a harder
287:
The fraction of the ordered molecules in polymer is characterized by the degree of crystallinity, which typically ranges between 10% and 80%. Higher values are only achieved in materials having small molecules, which are usually brittle, or in samples stored for long time at temperatures just under
259:
When polymers crystallize from an isotropic, bulk of melt or concentrated solution, the crystalline lamellae (10 to 20 nm in thickness) are typically organized into a spherulitic morphology as illustrated above. However, when polymer chains are confined in a space with dimensions of a few tens
246:
A very different process is precipitation; it uses a solvent which dissolves individual monomers but not the resulting polymer. When a certain degree of polymerization is reached, the polymerized and partially crystallized product precipitates out of the solution. The rate of crystallization can be
242:
Polymers can also be crystallized from a solution or upon evaporation of a solvent. This process depends on the degree of dilution: in dilute solutions, the molecular chains have no connection with each other and exist as a separate polymer coils in the solution. Increase in concentration which can
640:
materials meaning that under applied stress, their deformation increases with time (creep). The elastic properties of plastics are therefore distinguished according to the time scale of the testing to short-time behavior (such as tensile test which lasts minutes), shock loading, the behavior under
632:
As done in crystalline materials, particles can be added to semi-crystalline polymers to change the mechanical properties. In crystalline materials the addition of particles works to impede dislocation motion and strengthen the material. However, for many semi-crystalline polymers particle fillers
139:
Apart from the thermal mechanism, nucleation is strongly affected by impurities, dyes, plasticizers, fillers and other additives in the polymer. This is also referred to as heterogeneous nucleation. This effect is poorly understood and irregular, so that the same additive can promote nucleation in
616:
Above the glass transition temperature amorphous chains in a semi-crystalline polymer are ductile and are able to deform plastically. Crystalline regions of the polymer are linked by the amorphous regions. Tie molecules prevent the amorphous and crystalline phases from separating under an applied
544:
Regular arrangement of atoms and molecules produce sharp diffraction peaks whereas amorphous regions result in broad halos. The diffraction pattern of polymers usually contains a combination of both. Degree of crystallinity can be estimated by integrating the relative intensities of the peaks and
190:
and is suppressed at the top and bottom of the lamellae by the amorphous folded parts at those surfaces. In the case of a strong gradient, the growth has a unidirectional, dendritic character. However, if temperature distribution is isotropic and static then lamellae grow radially and form larger
222:
which partially aligns its molecules. Such alignment can be considered as crystallization and it affects the material properties. For example, the strength of the fiber is greatly increased in the longitudinal direction, and optical properties show large anisotropy along and perpendicular to the
60:
Polymers are composed of long molecular chains which form irregular, entangled coils in the melt. Some polymers retain such a disordered structure upon freezing and readily convert into amorphous solids. In other polymers, the chains rearrange upon freezing and form partly ordered regions with a
628:
Other defects, such as voids, occur in the semi-crystalline polymer under tensile stress and can drive the formation of the neck. The voids can be observed via small angle x-ray scattering. Unlike crazes these voids do not transfer stresses. Notably, cavitation is not observed under compressive
624:
After yielding, a neck is formed in the amorphous region and propagates down the sample length. During necking, the disordered chains align along the tensile direction, forming an ordered structure that demonstrates strengthening due to the molecular reorientation. The flow stress now increases
278:
topochemical polymerisation are generally crystalline. In many cases, the monomer to polymer transition occurs with the retention of crystallinity. Often one can determine the crystal structure of such polymers and the mechanism of polymerisation via single crystal X-ray diffraction. Since the
38:
is estimated by different analytical methods and it typically ranges between 10 and 80%, with crystallized polymers often called "semi-crystalline". The properties of semi-crystalline polymers are determined not only by the degree of crystallinity, but also by the size and orientation of the
649:
Crystalline polymers are usually opaque because of light scattering on the numerous boundaries between the crystalline and amorphous regions. The density of such boundaries is lower in polymers with very low crystallinity (amorphous polymer) or very high degree of crystalline polymers,
633:
weaken the material. It has been suggested that for particles to have a toughening effect in polymers the interparticle matrix ligament thickness must be smaller than a certain threshold. Crystalline polymers polypropylene and polyethylene display particle strengthening.
135:
starts with small, nanometer-sized areas where as a result of heat motion some chains or their segments occur parallel. Those seeds can either dissociate, if thermal motion destroys the molecular order, or grow further, if the grain size exceeds a certain critical value.
223:
fiber axis. Such anisotropy is more enhanced in presence of rod-like fillers such as carbon nanotubes, compared to spherical fillers. Polymer strength is increased not only by extrusion, but also by blow molding, which is used in the production of plastic tanks and
654:
polypropylene, which has crystallinity ~50%, is opaque. Crystallinity also affects dyeing of polymers: crystalline polymers are more difficult to stain than amorphous ones because the dye molecules penetrate through amorphous regions with greater ease.
579:. Polymers can crystallize through a variety of different regimes and unlike simple molecules, the polymer crystal lamellae have two very different surfaces. The two most prominent theories in polymer crystallization kinetics are the
99:
Whether or not polymers can crystallize depends on their molecular structure – presence of straight chains with regularly spaced side groups facilitates crystallization. For example, crystallization occurs much easier in
263:
The unique crystal orientation of confined polymers imparts anisotropic properties. In one example the large, in-plane polymer crystals reduce the gas permeability of nanolayered films by almost 2 orders of magnitude.
291:
Most methods of evaluating the degree of crystallinity assume a mixture of perfect crystalline and totally disordered areas; the transition areas are expected to amount to several percent. These methods include
172:. Higher temperatures destroy the molecular arrangement and below the glass transition temperature, the movement of molecular chains is frozen. Nevertheless, secondary crystallization can proceed even below T
1316:
515:
Crystalline areas are generally more densely packed than amorphous areas. This results in a higher density, up to 15% depending on the material. For example, polyamide 6 (nylon) has crystalline density
1496:
Bartczak, Z., Argon A.S., Weinberg, M. Toughness mechanism in semi-crystalline polymer blends: II. High-density polyethylene toughened with calcium carbonate filler particles. Polymer, 1999. 2347-2365.
571:
The methods used to determine the degree of crystallinity can be incorporated over time to measure the kinetics of crystallization. The most basic model for polymer crystallization kinetics comes from
140:
one polymer, but not in another. Many of the good nucleating agents are metal salts of organic acids, which themselves are crystalline at the solidification temperature of the polymer solidification.
621:
occurring on a large number of planes. Conversely, coarse slip is a large amount of slip on few planes. The yield stress is determined by the creation of dislocations and their resistance to motion.
34:
can crystallize upon cooling from melting, mechanical stretching or solvent evaporation. Crystallization affects optical, mechanical, thermal and chemical properties of the polymer. The degree of
1069:
Wang, Haopeng; Jong K. Keum; Anne
Hiltner; Eric Baer; Benny Freeman; Artur Rozanski; Andrzej Galeski (6 February 2009). "Confined Crystallization of Polyethylene Oxide in Nanolayer Assemblies".
176:, in the time scale of months and years. This process affects mechanical properties of the polymers and decreases their volume because of a more compact packing of aligned polymer chains.
965:
Patil, N; Balzano, L; Portale, G; Rastogi, S (July 2010). "A Study on the Chain−Particle
Interaction and Aspect Ratio of Nanoparticles on Structure Development of a Linear Polymer".
562:
crystalline and amorphous areas differ by the mobility of protons. The latter can be monitored through the line shape of NMR signals and used to estimate the degree of crystallinity.
952:
183:. The interaction strength depends on the distance between the parallel chain segments and it determines the mechanical and thermal properties of the polymer.
1269:
Kory, Max J.; Wörle, Michael; Weber, Thomas; Payamyar, Payam; van de Poll, Stan W.; Dshemuchadse, Julia; Trapp, Nils; Schlüter, A. Dieter (September 2014).
315:
In addition to the above integral methods, the distribution of crystalline and amorphous regions can be visualized with microscopic techniques, such as
742:
1469:
Pawlak, A., Galeski A,. Rozanski, A. Cavitation during deformation of semicrystalline polymers. Progress in
Polymer Science. (2014). 921-958
1031:
J. Lehmann (1966). "The observation of the crystallization of high polymer substances from the solution by nuclear magnetic resonance".
22:
is a process associated with partial alignment of their molecular chains. These chains fold together and form ordered regions called
156:
Crystal growth is achieved by the further addition of folded polymer chain segments and only occurs for temperatures below the
1571:
1544:
1517:
828:
782:
752:
650:
consequentially, the transparency is higher. For example, atactic polypropylene is usually amorphous and transparent while
536:
and compared with that released upon melting of the standard sample of the same material with known crystallization degree.
312:(NMR). The measured value depends on the method used, which is therefore quoted together with the degree of crystallinity.
1478:
Bowden, P.B., Young, R.J. Deformation
Mechanisms in Crystalline Polymers. Journal of Materials Science. (1974), 2034-2051.
1446:
1424:
1387:
1016:
941:
916:
896:
863:
533:
297:
1607:
320:
1330:
Luo, Liang; Wilhelm, Christopher; Sun, Aiwu; Grey, Clare P.; Lauher, Joseph W.; Goroff, Nancy S. (18 June 2008).
104:
than in the atactic polypropylene form. Atactic polymers crystallize when the side groups are very small, as in
1332:"Poly(diiododiacetylene): Preparation, Isolation, and Full Characterization of a Very Simple Poly(diacetylene)"
1120:
Hema, Kuntrapakam; Ravi, Arthi; Raju, Cijil; Pathan, Javed R.; Rai, Rishika; Sureshan, Kana M. (2021-03-29).
1271:"Gram-scale synthesis of two-dimensional polymer crystals and their structure analysis by X-ray diffraction"
955:
in the IWF Knowledge and Media gGmbH (videos and articles on the dendritic crystallization of polypropylene)
669:
165:
27:
524:= 1.08 g/cm). However, moisture which is often present in the sample does affect this type of measurement.
128:
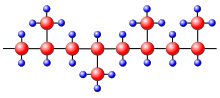
Lamellae form during crystallization from the melt. The arrow shows the direction of temperature gradient.
124:
1592:
268:
1597:
609:. Heat resistance is usually given for amorphous polymers just below the glass transition temperature.
532:
Additional energy is released upon melting a semicrystalline polymer. This energy can be measured with
412:
395:
316:
309:
248:
227:
bottles. Some polymers which do not crystallize from the melt, can be partially aligned by stretching.
224:
66:
1438:
Plastics in engineering applications: properties, processing and practical use of polymeric materials.
572:
477:
1121:
493:
429:
199:
70:
664:
231:
1534:
1507:
1436:
1377:
1224:"Single-Crystal-to-Single-Crystal (SCSC) Linear Polymerization of a Desymmetrized Anthraphane"
931:
886:
1561:
1414:
1223:
1176:
1008:
855:
818:
305:
772:
1078:
974:
700:
187:
47:
8:
606:
180:
23:
16:
Partial alignment of polymer molecular chains, resulting in "semi-crystalline" structures
1177:"Spontaneous Single-Crystal-to-Single-Crystal Evolution of Two Cross-Laminated Polymers"
1082:
978:
704:
288:
the melting point. The latter procedure is costly and is applied only in special cases.
186:
The growth of the crystalline regions preferably occurs in the direction of the largest
1251:
1204:
1157:
1102:
1048:
716:
152:
Schematic model of a spherulite. Black arrows indicate direction of molecular alignment
1602:
1567:
1540:
1513:
1442:
1420:
1383:
1359:
1351:
1298:
1290:
1243:
1196:
1161:
1149:
1141:
1094:
1012:
990:
937:
912:
892:
859:
824:
778:
748:
301:
208:
207:
The above mechanism considered crystallization from the melt, which is important for
1255:
1208:
1052:
1343:
1282:
1235:
1188:
1133:
1086:
1040:
982:
720:
708:
378:
1457:
Courtney, T. H. "Mechanical
Behavior of Materials". Waveland Press (2005), 392-396
247:
monitored by a technique which selectively probes the dissolved fraction, such as
1487:
Courtney, T. H.. Mechanical
Behavior of Materials. Waveland Press (2005), 392-396
1106:
637:
602:
580:
1317:"Topochemical polymerizations for the solid-state synthesis of organic polymers"
1122:"Topochemical polymerizations for the solid-state synthesis of organic polymers"
575:. The crystallization process of polymers does not always obey simple chemical
219:
770:
51:
The arrangement of molecular chains in amorphous and semicrystalline polymers.
1586:
1355:
1331:
1294:
1270:
1145:
994:
688:
576:
447:
157:
77:
35:
1090:
218:
In this process, the polymer is forced through, e.g., a nozzle that creates
1363:
1302:
1247:
1239:
1200:
1192:
1153:
1098:
740:
62:
230:
Some elastomers which are amorphous in the unstrained state undergo rapid
203:
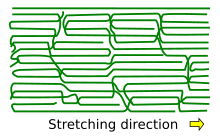
The arrangement of the molecule chains upon crystallization by stretching.
83:
854:
Georg Menges, Edmund
Haberstroh, Walter Michaeli, Ernst Schmachtenberg:
91:
1286:
1137:
1044:
641:
long-term and static loading, as well as the vibration-induced stress.
132:
1347:
986:
1068:
712:
651:
212:
105:
101:
74:
1401:
Fundamentals of
Polymer Science An Introductory Text, Second Edition
816:
1532:
328:
Degree of crystallinity (D, %) and densities of crystalline (ρ
211:
of plastic components. Another type of crystallization occurs upon
113:
1398:
293:
31:
1222:
Servalli, Marco; Trapp, Nils; Schlüter, A. Dieter (2018-10-09).
1559:
109:
148:
361:
108:
and don't crystallize in case of large substituents like in
771:
Linda C. Sawyer; David T. Grubb; Gregory F. Meyers (2008).
964:
885:
GW Becker, Ludwig
Bottenbruch, Rudolf Binsack, D. Braun:
586:
820:
Polymeric materials: structure, properties, applications
1376:
Gottfried W. Ehrenstein, Gabriela Riedel, Pia
Trawiel:
1268:
741:
Charles E. Carraher; Raymond Benedict Seymour (2003).
566:
1221:
1175:
Athiyarath, Vignesh; Sureshan, Kana M. (2019-01-08).
1505:
591:
26:, which compose larger spheroidal structures named
1465:
1463:
1174:
1119:
1399:Paul C. Painter; Michael M. Coleman (1997). "8".
1329:
1584:
691:(1952). "Morphology of crystallizing polymers".
237:
194:
1509:Introduction to Materials Science for Engineers
1460:
907:Wilbrand Woebcken, Klaus Stöckhert, HBP Gupta:
817:G. W. Ehrenstein; Richard P. Theriault (2001).
143:
55:
1536:Polymer chemistry: properties and applications
1533:Andrew J. Peacock; Allison R. Calhoun (2006).
267:
687:
179:The chains interact via various types of the
42:
1007:Michael Thielen, Klaus Hartwig, Peter Gust:
1563:Chemical principles of textile conservation
1370:
254:
1560:Ágnes Tímár-Balázsy; Dinah Eastop (1998).
1030:
1026:
1024:
926:
924:
282:
1379:Practice of thermal analysis of plastics.
605:deformation, i.e., the polymer begins to
554:degree of crystallinity can be estimated.
87:The structure of isotactic polypropylene.
1441:(in German) Vieweg+Teubner Verlag, 2008
1336:Journal of the American Chemical Society
933:Materials science and materials testing.
881:
879:
877:
875:
873:
871:
850:
848:
846:
844:
842:
840:
198:
147:
123:
90:
82:
46:
1407:
1181:Angewandte Chemie International Edition
1064:
1062:
1021:
1001:
921:
901:
888:Engineering Thermoplastics. Polyamides.
95:The structure of atactic polypropylene.
1585:
1429:
812:
810:
808:
806:
804:
802:
800:
798:
796:
794:
587:Properties of semicrystalline polymers
1566:. Butterworth-Heinemann. p. 11.
868:
837:
766:
764:
681:
644:
583:and Lauritzen-Hoffman Growth Theory.
1059:
744:Seymour/Carraher's polymer chemistry
736:
734:
732:
730:
1539:. Hanser Verlag. pp. 286–287.
1512:. Prentice Hall. pp. 168–169.
791:
567:Kinetics of polymer crystallization
520:= 1.24 g/cm and amorphous density ρ
13:
946:
761:
14:
1619:
1419:(in German) Hanser Verlag, 2006,
911:(in German) Hanser Verlag, 1998,
823:. Hanser Verlag. pp. 67–78.
727:
592:Thermal and mechanical properties
534:differential scanning calorimetry
298:differential scanning calorimetry
215:used in making fibers and films.
1009:Blow molding of plastic articles
891:(in German) Hanser Verlag, 1998
559:Nuclear magnetic resonance (NMR)
321:transmission electron microscopy
1553:
1526:
1499:
1490:
1481:
1472:
1451:
1392:
1323:
1309:
1262:
1215:
1168:
1113:
958:
936:Vieweg + Teubner Verlag, 2007,
1228:Chemistry - A European Journal
1:
1506:James F. Shackelford (2009).
1033:Colloid & Polymer Science
747:. CRC Press. pp. 43–45.
675:
238:Crystallization from solution
195:Crystallization by stretching
119:
670:Modeling of polymer crystals
166:glass transition temperature
144:Crystal growth from the melt
56:Solidification from the melt
7:
658:
269:Topochemical polymerization
20:Crystallization of polymers
10:
1624:
856:Plastics Materials Science
550:Infrared spectroscopy (IR)
413:Polybutylene terephthalate
396:Polyethylene terephthalate
317:polarized light microscopy
310:nuclear magnetic resonance
249:nuclear magnetic resonance
67:polyethylene terephthalate
43:Crystallization mechanisms
573:Hoffman nucleation theory
478:High-density polyethylene
1126:Chemical Society Reviews
494:Low-density polyethylene
255:Confined crystallization
1608:Liquid-solid separation
1091:10.1126/science.1164601
777:. Springer. p. 5.
430:Polytetrafluoroethylene
283:Degree of crystallinity
71:polytetrafluoroethylene
1240:10.1002/chem.201802513
1193:10.1002/anie.201812094
909:Plastics Encyclopedia.
665:Liquid-crystal polymer
204:
153:
129:
96:
88:
52:
1382:Hanser Verlag, 2003,
1011:Hanser Verlag, 2006,
858:Hanser Verlag, 2002,
463:atactic polypropylene
306:infrared spectroscopy
202:
151:
127:
94:
86:
50:
930:Wolfgang Weissbach:
512:Density measurements
188:temperature gradient
181:van der Waals forces
1234:(56): 15003–15012.
1083:2009Sci...323..757W
979:2010MaMol..43.6749P
705:1952Natur.169..913K
337:
158:melting temperature
1593:Chemical processes
1287:10.1038/nchem.2007
1138:10.1039/D0CS00840K
1045:10.1007/BF01553085
774:Polymer microscopy
645:Optical properties
336:, g/cm) polymers.
332:) and amorphous (ρ
327:
205:
154:
130:
97:
89:
53:
39:molecular chains.
1598:Phase transitions
1573:978-0-7506-2620-0
1546:978-1-56990-397-1
1519:978-0-13-601260-3
1413:Joachim Nentwig:
1348:10.1021/ja8011403
1342:(24): 7702–7709.
1077:(5915): 757–760.
987:10.1021/ma100636v
973:(16): 6749–6759.
830:978-1-56990-310-0
784:978-0-387-72627-4
754:978-0-8247-0806-1
699:(4309): 913–914.
541:X-ray diffraction
508:
507:
302:X-ray diffraction
234:upon stretching.
209:injection molding
1615:
1578:
1577:
1557:
1551:
1550:
1530:
1524:
1523:
1503:
1497:
1494:
1488:
1485:
1479:
1476:
1470:
1467:
1458:
1455:
1449:
1433:
1427:
1411:
1405:
1404:
1396:
1390:
1374:
1368:
1367:
1327:
1321:
1320:
1313:
1307:
1306:
1275:Nature Chemistry
1266:
1260:
1259:
1219:
1213:
1212:
1172:
1166:
1165:
1132:(6): 4062–4099.
1117:
1111:
1110:
1066:
1057:
1056:
1028:
1019:
1005:
999:
998:
962:
956:
950:
944:
928:
919:
905:
899:
883:
866:
852:
835:
834:
814:
789:
788:
768:
759:
758:
738:
725:
724:
713:10.1038/169913a0
685:
379:Polyoxymethylene
338:
326:
274:Polymers formed
1623:
1622:
1618:
1617:
1616:
1614:
1613:
1612:
1583:
1582:
1581:
1574:
1558:
1554:
1547:
1531:
1527:
1520:
1504:
1500:
1495:
1491:
1486:
1482:
1477:
1473:
1468:
1461:
1456:
1452:
1435:Martin Bonnet:
1434:
1430:
1412:
1408:
1397:
1393:
1375:
1371:
1328:
1324:
1315:
1314:
1310:
1267:
1263:
1220:
1216:
1173:
1169:
1118:
1114:
1067:
1060:
1029:
1022:
1006:
1002:
963:
959:
951:
947:
929:
922:
906:
902:
884:
869:
853:
838:
831:
815:
792:
785:
769:
762:
755:
739:
728:
686:
682:
678:
661:
647:
600:
594:
589:
581:Avrami equation
569:
523:
519:
509:
356:
350:
335:
331:
285:
272:
257:
240:
232:crystallization
197:
175:
171:
163:
146:
122:
58:
45:
17:
12:
11:
5:
1621:
1611:
1610:
1605:
1600:
1595:
1580:
1579:
1572:
1552:
1545:
1525:
1518:
1498:
1489:
1480:
1471:
1459:
1450:
1428:
1406:
1391:
1369:
1322:
1308:
1281:(9): 779–784.
1261:
1214:
1187:(2): 612–617.
1167:
1112:
1058:
1039:(2): 167–168.
1020:
1000:
967:Macromolecules
957:
945:
920:
900:
867:
836:
829:
790:
783:
760:
753:
726:
679:
677:
674:
673:
672:
667:
660:
657:
646:
643:
598:
593:
590:
588:
585:
577:rate equations
568:
565:
564:
563:
560:
556:
555:
551:
547:
546:
542:
538:
537:
530:
526:
525:
521:
517:
513:
506:
505:
502:
499:
496:
490:
489:
486:
483:
480:
474:
473:
470:
467:
464:
460:
459:
456:
453:
450:
443:
442:
439:
436:
433:
426:
425:
422:
419:
416:
409:
408:
405:
402:
399:
392:
391:
388:
385:
382:
375:
374:
371:
368:
365:
364:(PA66 and PA6)
358:
357:
354:
351:
348:
345:
342:
333:
329:
325:
284:
281:
271:
266:
256:
253:
239:
236:
220:tensile stress
196:
193:
173:
169:
164:and above the
161:
145:
142:
121:
118:
57:
54:
44:
41:
15:
9:
6:
4:
3:
2:
1620:
1609:
1606:
1604:
1601:
1599:
1596:
1594:
1591:
1590:
1588:
1575:
1569:
1565:
1564:
1556:
1548:
1542:
1538:
1537:
1529:
1521:
1515:
1511:
1510:
1502:
1493:
1484:
1475:
1466:
1464:
1454:
1448:
1447:3-8348-0349-9
1444:
1440:
1439:
1432:
1426:
1425:3-446-40390-6
1422:
1418:
1417:
1416:Plastic films
1410:
1402:
1395:
1389:
1388:3-446-22340-1
1385:
1381:
1380:
1373:
1365:
1361:
1357:
1353:
1349:
1345:
1341:
1337:
1333:
1326:
1318:
1312:
1304:
1300:
1296:
1292:
1288:
1284:
1280:
1276:
1272:
1265:
1257:
1253:
1249:
1245:
1241:
1237:
1233:
1229:
1225:
1218:
1210:
1206:
1202:
1198:
1194:
1190:
1186:
1182:
1178:
1171:
1163:
1159:
1155:
1151:
1147:
1143:
1139:
1135:
1131:
1127:
1123:
1116:
1108:
1104:
1100:
1096:
1092:
1088:
1084:
1080:
1076:
1072:
1065:
1063:
1054:
1050:
1046:
1042:
1038:
1034:
1027:
1025:
1018:
1017:3-446-22671-0
1014:
1010:
1004:
996:
992:
988:
984:
980:
976:
972:
968:
961:
954:
949:
943:
942:3-8348-0295-6
939:
935:
934:
927:
925:
918:
917:3-446-17969-0
914:
910:
904:
898:
897:3-446-16486-3
894:
890:
889:
882:
880:
878:
876:
874:
872:
865:
864:3-446-21257-4
861:
857:
851:
849:
847:
845:
843:
841:
832:
826:
822:
821:
813:
811:
809:
807:
805:
803:
801:
799:
797:
795:
786:
780:
776:
775:
767:
765:
756:
750:
746:
745:
737:
735:
733:
731:
722:
718:
714:
710:
706:
702:
698:
694:
690:
689:Andrew Keller
684:
680:
671:
668:
666:
663:
662:
656:
653:
642:
639:
636:Plastics are
634:
630:
626:
622:
618:
614:
610:
608:
604:
601:results in a
584:
582:
578:
574:
561:
558:
557:
552:
549:
548:
543:
540:
539:
535:
531:
528:
527:
514:
511:
510:
503:
500:
497:
495:
492:
491:
487:
484:
481:
479:
476:
475:
471:
468:
465:
462:
461:
457:
454:
451:
449:
448:polypropylene
445:
444:
440:
437:
434:
431:
428:
427:
423:
420:
417:
414:
411:
410:
406:
403:
400:
397:
394:
393:
389:
386:
383:
380:
377:
376:
372:
369:
366:
363:
360:
359:
352:
346:
343:
340:
339:
324:
322:
318:
313:
311:
307:
303:
299:
296:measurement,
295:
289:
280:
277:
270:
265:
261:
252:
250:
244:
235:
233:
228:
226:
221:
216:
214:
210:
201:
192:
189:
184:
182:
177:
167:
159:
150:
141:
137:
134:
126:
117:
115:
111:
107:
103:
93:
85:
81:
79:
78:polypropylene
76:
72:
68:
64:
49:
40:
37:
36:crystallinity
33:
29:
25:
21:
1562:
1555:
1535:
1528:
1508:
1501:
1492:
1483:
1474:
1453:
1437:
1431:
1415:
1409:
1403:. CRC Press.
1400:
1394:
1378:
1372:
1339:
1335:
1325:
1311:
1278:
1274:
1264:
1231:
1227:
1217:
1184:
1180:
1170:
1129:
1125:
1115:
1074:
1070:
1036:
1032:
1003:
970:
966:
960:
948:
932:
908:
903:
887:
819:
773:
743:
696:
692:
683:
652:syndiotactic
648:
638:viscoelastic
635:
631:
627:
623:
619:
615:
611:
603:viscoelastic
595:
570:
314:
290:
286:
275:
273:
262:
258:
245:
241:
229:
217:
206:
185:
178:
155:
138:
131:
98:
63:polyethylene
59:
19:
18:
529:Calorimetry
28:spherulites
1587:Categories
676:References
446:isotactic
133:Nucleation
120:Nucleation
73:(PTFE) or
1356:0002-7863
1295:1755-4349
1162:231819465
1146:1460-4744
995:0024-9297
213:extrusion
114:silicones
106:polyvinyl
102:isotactic
75:isotactic
1603:Polymers
1364:18489101
1303:25143212
1256:51599257
1248:29984526
1209:53945431
1201:30461147
1154:33543741
1099:19197057
1053:96640893
953:Dendrite
659:See also
32:Polymers
24:lamellae
1079:Bibcode
1071:Science
975:Bibcode
721:4255757
701:Bibcode
341:Polymer
304:(XRD),
300:(DSC),
294:density
69:(PET),
1570:
1543:
1516:
1445:
1423:
1386:
1362:
1354:
1301:
1293:
1254:
1246:
1207:
1199:
1160:
1152:
1144:
1105:
1097:
1051:
1015:
993:
940:
915:
895:
862:
827:
781:
751:
719:
693:Nature
545:halos.
432:(PTFE)
110:rubber
80:(PP).
65:(PE),
1252:S2CID
1205:S2CID
1158:S2CID
1107:19276
1103:S2CID
1049:S2CID
717:S2CID
607:creep
504:0.85
498:45–55
488:0.85
482:70–80
458:0.85
452:70–80
441:2.00
435:60–80
418:40–50
415:(PBT)
407:1.33
401:30–40
398:(PET)
390:1.28
384:70–80
381:(POM)
373:1.08
367:35–45
362:Nylon
1568:ISBN
1541:ISBN
1514:ISBN
1443:ISBN
1421:ISBN
1384:ISBN
1360:PMID
1352:ISSN
1299:PMID
1291:ISSN
1244:PMID
1197:PMID
1150:PMID
1142:ISSN
1095:PMID
1013:ISBN
991:ISSN
938:ISBN
913:ISBN
893:ISBN
860:ISBN
825:ISBN
779:ISBN
749:ISBN
455:0.95
438:2.35
404:1.50
387:1.54
370:1.24
319:and
308:and
1344:doi
1340:130
1283:doi
1236:doi
1189:doi
1134:doi
1087:doi
1075:323
1041:doi
1037:212
983:doi
709:doi
697:169
501:1.0
485:1.0
276:via
225:PET
112:or
1589::
1462:^
1358:.
1350:.
1338:.
1334:.
1297:.
1289:.
1277:.
1273:.
1250:.
1242:.
1232:24
1230:.
1226:.
1203:.
1195:.
1185:58
1183:.
1179:.
1156:.
1148:.
1140:.
1130:50
1128:.
1124:.
1101:.
1093:.
1085:.
1073:.
1061:^
1047:.
1035:.
1023:^
989:.
981:.
971:43
969:.
923:^
870:^
839:^
793:^
763:^
729:^
715:.
707:.
695:.
472:–
466:~0
424:–
323:.
251:.
116:.
30:.
1576:.
1549:.
1522:.
1366:.
1346::
1319:.
1305:.
1285::
1279:6
1258:.
1238::
1211:.
1191::
1164:.
1136::
1109:.
1089::
1081::
1055:.
1043::
997:.
985::
977::
833:.
787:.
757:.
723:.
711::
703::
599:g
597:T
522:a
518:c
516:ρ
469:–
421:–
355:a
353:ρ
349:c
347:ρ
344:D
334:a
330:c
174:g
170:g
168:T
162:m
160:T
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.