937:
20:
175:
624:
1583:
842:
957:
724:), and methyl cation has only been unambiguously identified in the gas phase. In most, if not all cases, the ground state of alleged primary carbenium ions consist of bridged structures in which positive charge is shared by two or more carbon atoms and are better described as side-protonated alkenes, edge-protonated cyclopropanes, or corner-protonated cyclopropanes rather than true primary cations. The simple ethyl cation,
1175:
1136:
167:, like water, alcohols, carboxylates, azide, and halide ions, to form the addition product. Strongly basic nucleophiles, especially hindered ones, favor elimination over addition. Because even weak nucleophiles will react with carbocations, most can only be directly observed or isolated in non-nucleophilic media like
631:-butyl cation. The stabilizing interaction can be depicted as an orbital interaction or by resonance structures involving "no-bond" resonance forms. (For clarity, a dashed line is used to show that the hydrogen atom is still attached, although the formal C–H bond order in the hyperconjugative structure is zero.)
1181:
Two hydrogen atoms bonded to one carbon lie in a plane perpendicular to the benzene ring. The arenium ion is no longer an aromatic species; however it is relatively stable due to delocalization: the positive charge is delocalized over 5 carbon atoms. Also contributing to the stability of arenium ions
940:
The sp2 lone pair of molecule A is oriented such that it forms sufficient orbital overlap with the empty p orbital of the carbonation to allow the formation of a π bond, sequestering the carbonation in a contributing resonance structure. The lone pair of molecule B is rotated 90° with respect to the
932:
ions have important secondary canonical forms (resonance structures) in which carbon bears a positive charge. As such, they are carbocations according to the IUPAC definition although some chemists do not regard them to be "true" carbocations, as their most important resonance contributors carry the
1328:
Based on hydride ion affinity, the parent vinyl cation is less stable than even a primary sp-hybridized carbocation, while an α alkyl-substituted vinyl cation has a stability that is comparable to the latter. Hence, vinyl cations are relatively uncommon intermediates. They can be generated by the
740:
has been demonstrated experimentally and computationally to be bridged and can be thought of as a symmetrically protonated ethylene molecule. The same is true for higher homologues like 1-propyl and 1-butyl cations. Neopentyl derivatives are thought to ionize with concomitant migration of a methyl
138:
rapidly rearrange to give the 1-methyl-1-cyclopentyl cation. This fact often complicates synthetic pathways. For example, when 3-pentanol is heated with aqueous HCl, the initially formed 3-pentyl carbocation rearranges to a statistical mixture of the 3-pentyl and 2-pentyl. These cations react with
110:
readily. For example, when pentan-3-ol is heated with aqueous HCl, the initially formed 3-pentyl carbocation rearranges to a mixture of the 3-pentyl and 2-pentyl. These cations react with chloride ion to produce 3-chloropentane and 2-chloropentane in a ratio of approximately 1:2. Migration of an
1344:
1(vinyl)) and as intermediates in the electrophilic addition reactions of arylalkynes. With the exception of the parent vinyl cation, which is believed to be a bridged species, and geometrically constrained cyclic vinyl cations, most vinyl cations take on sp hybridization and are linear.
122:). In especially favorable cases like the 2-norbornyl cation, hydrogen shifts may still take place at rates fast enough to interfere with X-ray crystallography at 86 K (−187 °C). Typically, carbocations will rearrange to give a tertiary isomer. For instance, all isomers of
2481:(1972). "Stable carbocations. CXVIII. General concept and structure of carbocations based on differentiation of trivalent (classical) carbenium ions from three-center bound penta- of tetracoordinated (nonclassical) carbonium ions. Role of carbocations in electrophilic reactions".
1131:
These varying cation stabilities, depending on the number of π electrons in the ring system, can furthermore be crucial factors in reaction kinetics. The formation of an aromatic carbocation is much faster than the formation of an anti-aromatic or open-chain carbocation.
1572:
941:
empty p orbital of the carbonation, demonstrated by the Newman projection (bottom right). Without proper orbital overlap, the nitrogen lone pair cannot donate into the carbocation's empty p orbital. Thus, the carbocation in molecule B is not resonance-stabilized.
2197:
George A. Olah and
Joachim Lukas (1967), "Stable Carbonium Ions. XLVII. Alkylcarbonium ion formation from alkanes via hydride (alkide) ion abstraction in fluorosulfonic acid-antimony pentafluoride-sulfuryl chlorofluoride solution".
1112:. Although less stable than the tropylium cation, this carbenium ion can also form salts at room temperature. Solutions of such salts were exhibit conventional spectroscopic and chemical properties. The cyclopropenium cation (
2126:
Schultz, Jocelyn C.; Houle, F. A.; Beauchamp, J. L. (July 1984). "Photoelectron spectroscopy of 1-propyl, 1-butyl, isobutyl, neopentyl, and 2-butyl radicals: free radical precursors to high-energy carbonium ion isomers".
562:
Since carbenium ions can be highly reactive, a major consideration is their stability. The stability of carbenium ions correlates with the electron-donating properties of the substituents. Trialkylcarbenium ions, such as
2162:
Yamataka, Hiroshi; Ando, Takashi; Nagase, Shigeru; Hanamura, Mitsuyasu; Morokuma, Keiji (February 1984). "Ab initio MO calculations of isotope effects in model processes of neopentyl ester solvolysis".
1989:
Olah, George A.; O'Brien, Daniel H.; White, Anthony
Mallinson. (October 1967). "Stable carbonium ions. LII. Protonated esters and their cleavage in fluorosulfonic acid-antimony pentafluoride solution".
918:(trityl) cation, are particularly stable. For the same reasons, the partial p character of strained C–C bonds in cyclopropyl groups also allows for donation of electron density and stabilizes the
1400:
914:
are more stable than most other carbenium ions due to donation of electron density from π systems to the cationic center. The doubly- and triply-benzylic carbocations, diphenylcarbenium and
1676:
Scholz, Franziska; Himmel, Daniel; Scherer, Harald; Krossing, Ingo (2013). "Superacidic or Not…︁? Synthesis, Characterisation, and
Acidity of the Room-Temperature Ionic Liquid [C(CH
192:
The stability order of carbocations, from most stable to least stable as reflected by hydride ion affinity (HIA) values, are as follows (HIA values in kcal/mol in parentheses):
1266:
1265:=O. It is an acyl carbocation, but the actual structure has the oxygen and carbon linked by a triple bond. Such species are common reactive intermediates, for example, in the
1348:
Aryl cations are less stable than vinyl cations due to the ring-enforced distortion to a nonlinear geometry and approximately sp-character of the unoccupied orbital. Only
2557:"Isolating Benzenium Ion Salts" Christopher A. Reed, Kee-Chan Kim, Evgenii S. Stoyanov, Daniel Stasko, Fook S. Tham, Leonard J. Mueller, and Peter D. W. Boyd
713:
Primary carbocations in the solution phase, even as transient intermediates (the ethyl cation has been proposed for reactions in 99.9% sulfuric acid and in
2601:
Chevrier, B.; Le
Carpentier, J. M.; Weiss, R. (1972). "Synthesis of two crystalline species of the Friedel–Crafts intermediate antimony pentachloride-
2729:
2326:
1128:), although somewhat destabilized by angle strain, is still clearly stabilized by aromaticity when compared to its open-chain analog, allyl cation.
115:
in excess of 10 s at ambient temperature and still takes place rapidly (compared to the NMR timescale) at temperatures as low as −120 °C (
1593:
Carbenium ions are so integrated into organic chemistry that a full inventory of their commercially useful reactions would be long. For example,
1798:
2700:
Rahimi, Nazi; Karimzadeh, Ramin (2011). "Catalytic cracking of hydrocarbons over modified ZSM-5 zeolites to produce light olefins: A review".
51:, which is a general term for diamagnetic carbon-based cations. In parallel with carbenium ions is another subset of carbocations, the
2665:
Angelini, Giancarlo; Hanack, Michael; Vermehren, Jan; Speranza, Maurizio (1988-02-17). "Generation and trapping of an alkynyl cation".
1616:(LABs) illustrates the behaviour of secondary carbenium ions. The alkylation is initiated by strong acids. LABs are a key precursor to
1057:
In 1891 G. Merling obtained a water-soluble salt from a reaction of cycloheptatriene and bromine. The structure was elucidated by
695:
tertiary cations are stable and many are directly observable in superacid media. The stabilization by alkyl groups is explained by
1859:
1567:{\displaystyle {\ce {RC#CT -> + + e-}}+{\bar {\nu }}_{e}\longrightarrow {\ce {RC#C+ + ^{3}He + e-}}+{\bar {\nu }}_{e}}
1158:
2371:
2746:
2584:
1907:
2641:
2303:
2270:
2102:
2034:
1965:
1932:
1820:
1770:
1736:
118:
2434:"Aromaticity as a Cornerstone of Heterocyclic Chemistry" Alexandru T. Balaban, Daniela C. Oniciu, Alan R. Katritzky
2773:
699:. The donation of electron density from a β C-H or C-C bond into the unoccupied p orbital of the carbocation (a σ
2069:
607:. Carbenium ions can also be stabilized by conjugation to double bonds giving allyl cations, which enjoy some
1386:) and cannot be generated by purely chemical means. They can, however, be generated radiochemically via the
140:
2515:"A Quantum Mechanical Investigation of the Orientation of Substituents in Aromatic Molecules" G. W. Wheland
639:. This trend can be inferred by the hydride ion affinity values (231, 246, 273, and 312 kcal/mol for
1849:
1002:
148:
1058:
1340:). They have been implicated as intermediates in some vinyl substitution reactions (designated as S
1635:
1014:
144:
98:
ion, are relatively stable due to the positive charge being delocalised between the carbon atoms.
608:
936:
915:
773:
107:
745:); thus, in most if not all cases, a discrete neopentyl cation is not believed to be involved.
583:
cannot. An analogous situation applies to triarylcarbenium ions: salts of triphenylcarbenium
2778:
2545:
1274:
1239:
1051:
859:
742:
139:
chloride ion to produce about one third 3-chloropentane and two thirds 2-chloropentane. The
2605:-toluoyl chloride. Crystal structures of the donor–acceptor complex and of the ionic salt".
1873:
Hansjörg Grützmacher, Christina M. Marchand (1997), "Heteroatom stabilized carbenium ions",
174:
1329:
ionization of a vinyl electrophile, provided the leaving group is sufficiently good (e.g.,
152:
611:. This situation is illustrated by the isolation of protonated benzene. Lone-pair bearing
8:
1613:
1215:
789:
1033:
111:
alkyl group to form a new carbocationic center is also observed. This often occurs with
19:
2478:
1628:
1624:
1598:
1594:
1356:
1088:
845:
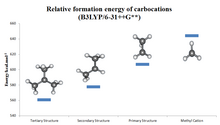
Order of stability of examples of tertiary (III), secondary (II), and primary (I) alkyl
1886:
2742:
2682:
2647:
2637:
2559:
2517:
2498:
2309:
2299:
2276:
2266:
2243:
2180:
2144:
2108:
2098:
2075:
2065:
2059:
2040:
2030:
2007:
1971:
1961:
1938:
1928:
1903:
1855:
1826:
1816:
1776:
1766:
1732:
1709:
623:
2458:"Cyclopropenyl Cation. Synthesis and Characterization." R. Breslow and J. T. Groves
2336:
2768:
2734:
2709:
2674:
2614:
2568:
2526:
2490:
2443:
2419:
2395:
2340:
2331:
2235:
2206:
2172:
2136:
1999:
1882:
1701:
1270:
1174:
1006:
951:
696:
95:
40:
2467:
1359:
salts is a good enough leaving group for the chemical generation of aryl cations.
2713:
2375:
1309:
603:), and those with amine substituents so robust that they are used as dyes, e.g.
1277:. Salts containing acylium ions can be generated by removal of the halide from
1234:). The benzenium salt is crystalline with thermal stability up to 150 °C.
604:
2399:
2064:. Richardson, Kathleen Schueller. (3rd ed.). New York: Harper & Row.
2762:
2738:
2727:
Gessner, Thomas; Mayer, Udo (2000). "Triarylmethane and
Diarylmethane Dyes".
2686:
2651:
2502:
2368:
2335:, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "
2313:
2280:
2184:
2148:
2112:
2044:
2011:
1975:
1942:
1830:
1780:
1726:
1656:
1639:
1305:
112:
52:
44:
2344:
2298:. Sundberg, Richard J. (5th ed.). New York: Springer. p. 426-427.
2265:. Sundberg, Richard J. (5th ed.). New York: Springer. p. 300-301.
2097:. Sundberg, Richard J. (5th ed.). New York: Springer. p. 300-301.
2079:
1927:. Sundberg, Richard J. (5th ed.). New York: Springer. p. 300-301.
2247:
2239:
1713:
1705:
1651:
1609:
1582:
1313:
1254:
is a cation with the formula RCO. The structure is described as R−C≡O or R−
1065:
925:
80:
1182:
is the energy gain resulting from the strong C-e bond (E = electrophile).
59:. In carbenium ions charge is localized. They are isoelectronic with mono
2410:"The Cycloheptatrienylium (Tropylium) Ion" W. von E. Doering, L. H. Knox
1845:
1278:
1251:
1235:
1148:
956:
841:
164:
90:
Carbenium ions are generally highly reactive due to having an incomplete
48:
2678:
2636:. Sundberg, Richard J. (5th ed.). New York: Springer. p. 436.
2618:
2589:
2530:
2494:
2423:
2210:
2176:
2140:
2003:
1815:. Sundberg, Richard J. (5th ed.). New York: Springer. p. 436.
1765:. Sundberg, Richard J. (5th ed.). New York: Springer. p. 440.
178:
Relative formation energy of carbocations from computational calculation
1605:
1387:
1214:
can be isolated as a stable compound when benzene is protonated by the
1157:
is a cyclohexadienyl cation that appears as a reactive intermediate in
863:
769:
612:
91:
2572:
2447:
1617:
1589:(hydrochloride salt), a commercial dye that contains a carbenium ion.
1374:(hydride ion affinity 386 kcal/mol versus 312 kcal/mol for
806:
168:
933:
formal positive charge on an oxygen or nitrogen atom, respectively.
1586:
1362:
Alkynyl cations are extremely unstable, much less stable than even
1135:
1021:
998:
969:
635:
The stability of alkyl-substituted carbocations follows the order
1391:
1186:
1010:
994:
929:
754:
196:
Hydride ion affinity (HIA) as a measure of carbocation stability
1851:
Advanced
Organic Chemistry: Reactions, Mechanisms, and Structure
43:
with carbon atom having three covalent bonds, and it bears a +1
2664:
1317:
1037:
890:
750:
710:-butyl, and cyclopentyl cations have been observed in solution.
600:
60:
36:
1312:. Acylium cations are characteristic fragments observed in EI-
703:→ p interaction) allows the positive charge to be delocalized.
1843:
1044:
1041:
868:
706:
Secondary cations are usually transient. Only the isopropyl,
2634:
Advanced
Organic Chemistry: Part A: Structure and Mechanisms
2296:
Advanced
Organic Chemistry: Part A: Structure and Mechanisms
2263:
Advanced
Organic Chemistry: Part A: Structure and Mechanisms
2095:
Advanced
Organic Chemistry: Part A: Structure and Mechanisms
1958:
Perspectives on structure and mechanism in organic chemistry
1925:
Advanced Organic Chemistry: Part A: Structure and Mechanisms
1813:
Advanced Organic Chemistry: Part A: Structure and Mechanisms
1763:
Advanced Organic Chemistry: Part A: Structure and Mechanisms
627:
Hyperconjugation by neighboring alkyl groups stabilizes the
2029:. Sundberg, Richard J. (5th ed.). New York: Springer.
1854:(6th ed.), New York: Wiley-Interscience, p. 239,
1675:
155:
to give the alkylated product) is more frequently applied.
94:
of electrons; however, certain carbenium ions, such as the
2600:
2161:
1054:
in which each carbon carries part of the positive charge.
1729:
Mechanism and Theory in Organic Chemistry, Second Edition
1727:
Thomas H. Lowery; Kathleen Schueller Richardson (1981).
1242:
are consistent with a cyclohexadienyl cation structure.
862:
by a carbon–carbon double bond or by the lone pair of a
1087:
Another aromatic carbenium ion is the cyclopropenyl or
691:). The effect of alkyl substitution is a strong one:
2222:
Kato, Tsuyoshi; Reed, Christopher A. (2004). "Putting
2125:
1403:
1161:. For historic reasons this complex is also called a
1001:). Salts of the tropylium cation can be stable, e.g.
1988:
27:-butyl cation is a relatively stable carbenium ion.
1323:
143:suffers from this limitation; for this reason, the
1566:
2760:
2699:
1897:
2730:Ullmann's Encyclopedia of Industrial Chemistry
2388:Berichte der deutschen chemischen Gesellschaft
1898:Anslyn, Eric V.; Dougherty, Dennis A. (2000).
1304:The C–O distance in these cations is near 1.1
1084:) is destabilized by some 40 kcal/mol.
2542:A guidebook to mechanism in organic chemistry
749:Carbenium ions can be prepared directly from
2726:
1134:
1050:The structure shown is a composite of seven
960:Ball-and-stick model of the tropylium cation
853:
1960:(2nd ed.). Hoboken, N.J.: John Wiley.
1902:. Sausalito, CA: University Science Books.
849:, as well as the methyl cation (far right).
945:
182:
163:Carbocations are susceptible to attack by
2061:Mechanism and theory in organic chemistry
2667:Journal of the American Chemical Society
2483:Journal of the American Chemical Society
2471:
2221:
2129:Journal of the American Chemical Society
1992:Journal of the American Chemical Society
1581:
1577:
955:
935:
840:
622:
173:
47:. Carbenium ions are a major subset of
18:
2228:Angewandte Chemie International Edition
1955:
1797:was invoked but never defined (see the
1185:The smallest arenium ion is protonated
1036:of aromaticity. It can coordinate as a
2761:
1601:involves carbenium ion intermediates.
39:with the structure RR′R″C, that is, a
2631:
2293:
2260:
2092:
2057:
2024:
1922:
1867:
1810:
1760:
993:. Its name derives from the molecule
2477:
2386:Merling, G. (1891), "Ueber Tropin".
2191:
866:adjacent to the ionized carbon. The
809:into the trimethylcarbenium cation,
2720:
1792:
1159:electrophilic aromatic substitution
858:A carbocation may be stabilized by
768:, with a strong acid. For example,
158:
13:
2585:Compendium of Chemical Terminology
2332:Compendium of Chemical Terminology
1634:Acylium ions are intermediates in
14:
2790:
1900:Modern Physical Organic Chemistry
1787:
1024:ion; it also has 6 π-electrons (4
101:
2165:The Journal of Organic Chemistry
1793:Cite error: The named reference
1731:. Harper and Rowe. p. 396.
1324:Vinyl and alkynyl carbenium ions
1173:
1032: = 1), which fulfills
618:
2693:
2658:
2625:
2594:
2577:
2551:
2535:
2509:
2452:
2428:
2404:
2380:
2349:
2320:
2287:
2254:
2215:
2155:
2119:
2086:
2051:
2018:
1982:
1949:
1916:
1245:
1142:
997:(itself named for the molecule
615:also stabilize carbenium ions.
74:
1891:
1837:
1804:
1754:
1745:
1720:
1694:Chemistry – A European Journal
1669:
1552:
1491:
1479:
1445:
1421:
1416:
922:(cyclopropylcarbinyl) cation.
637:3° > 2° > 1° > methyl
1:
1887:10.1016/S0010-8545(97)00043-X
1662:
575:, are isolable as salts, but
85:
2714:10.1016/j.apcata.2011.03.009
2702:Applied Catalysis A: General
2226:-Butyl Cation in a Bottle".
1308:, even shorter than that in
187:
7:
1645:
1003:tropylium tetrafluoroborate
10:
2795:
2632:Carey, Francis A. (2007).
2355:"Tropylium tetrafluorate"
2294:Carey, Francis A. (2007).
2261:Carey, Francis A. (2007).
2093:Carey, Francis A. (2007).
2027:Advanced Organic Chemistry
2025:Carey, Francis A. (2007).
1956:Carroll, Felix A. (2010).
1923:Carey, Francis A. (2007).
1811:Carey, Francis A. (2007).
1761:Carey, Francis A. (2007).
1146:
949:
599:are readily isolable (see
78:
2400:10.1002/cber.189102402151
2058:Lowry, Thomas H. (1987).
1636:Friedel-Crafts acylations
1527:
1267:Friedel−Crafts acylations
1068:cyclopentadienyl cation (
972:species with the formula
854:Extra stabilizing effects
141:Friedel–Crafts alkylation
106:Carbenium ions sometimes
2739:10.1002/14356007.a27_179
2357:Organic Syntheses, Coll.
1522:
1516:
1020:It is a planar, cyclic,
1015:phosphorus pentachloride
2733:. Weinheim: Wiley-VCH.
2345:10.1351/goldbook.M04002
1064:On the other hand, the
946:Aromatic carbenium ions
609:resonance stabilization
183:Types of carbenium ions
2774:Reactive intermediates
2363:, p.1138 (1973); Vol.
2240:10.1002/anie.200453931
1706:10.1002/chem.201203260
1590:
1568:
1139:
1052:resonance contributors
1028: + 2, where
1005:. It can be made from
961:
942:
850:
774:antimony pentafluoride
632:
179:
28:
1585:
1578:Selected applications
1569:
1275:Hayashi rearrangement
1240:X-ray crystallography
1138:
959:
939:
844:
743:anchimeric assistance
626:
177:
119:Wagner-Meerwein shift
22:
1401:
1163:Wheland intermediate
153:Clemmensen reduction
2679:10.1021/ja00212a052
2619:10.1021/ja00771a031
2531:10.1021/ja01256a047
2495:10.1021/ja00758a020
2424:10.1021/ja01641a027
2418:(12), pp 3203–3206
2211:10.1021/ja00994a030
2177:10.1021/jo00178a010
2141:10.1021/ja00326a006
2004:10.1021/ja00998a036
1844:Smith, Michael B.;
1629:triarylmethane dyes
1623:Derivatives of the
1614:linear alkylbenzene
1269:also in many other
1216:carborane superacid
790:fluorosulfuric acid
197:
2374:2012-08-29 at the
1625:triphenylcarbenium
1599:petroleum refining
1597:, a major step in
1595:catalytic cracking
1591:
1564:
1140:
1089:cyclopropenium ion
1061:and Knox in 1954.
1009:(tropylidene) and
962:
943:
916:triphenylcarbenium
851:
633:
195:
180:
55:with the formula R
29:
2613:(16): 5718–5723.
2573:10.1021/ja027336o
2560:J. Am. Chem. Soc.
2518:J. Am. Chem. Soc.
2460:J. Am. Chem. Soc.
2448:10.1021/cr0306790
2412:J. Am. Chem. Soc.
2234:(22): 2908–2911.
2200:J. Am. Chem. Soc.
2135:(14): 3917–3927.
1998:(22): 5694–5700.
1875:Coord. Chem. Rev.
1861:978-0-471-72091-1
1555:
1534:
1523:
1521:
1520:
1519:
1506:
1497:
1482:
1461:
1444:
1435:
1426:
1415:
1407:
1271:organic reactions
920:cyclopropylmethyl
560:
559:
2786:
2753:
2752:
2724:
2718:
2717:
2697:
2691:
2690:
2673:(4): 1298–1299.
2662:
2656:
2655:
2629:
2623:
2622:
2607:J. Am. Chem. Soc
2598:
2592:
2581:
2575:
2555:
2549:
2539:
2533:
2513:
2507:
2506:
2475:
2469:
2456:
2450:
2432:
2426:
2408:
2402:
2384:
2378:
2367:, p.101 (1963).
2353:
2347:
2324:
2318:
2317:
2291:
2285:
2284:
2258:
2252:
2251:
2219:
2213:
2205:(18), 4739–4744
2195:
2189:
2188:
2159:
2153:
2152:
2123:
2117:
2116:
2090:
2084:
2083:
2055:
2049:
2048:
2022:
2016:
2015:
1986:
1980:
1979:
1953:
1947:
1946:
1920:
1914:
1913:
1895:
1889:
1871:
1865:
1864:
1841:
1835:
1834:
1808:
1802:
1796:
1791:
1785:
1784:
1758:
1752:
1749:
1743:
1742:
1724:
1718:
1717:
1673:
1608:of benzene with
1573:
1571:
1570:
1565:
1563:
1562:
1557:
1556:
1548:
1541:
1540:
1539:
1532:
1517:
1512:
1511:
1504:
1502:
1495:
1490:
1489:
1484:
1483:
1475:
1468:
1467:
1466:
1459:
1454:
1453:
1448:
1442:
1441:
1440:
1433:
1431:
1424:
1413:
1412:
1405:
1385:
1384:
1383:
1373:
1372:
1371:
1354:
1339:
1332:
1300:
1299:
1298:
1264:
1263:
1262:
1259:
1209:
1208:
1207:
1199:
1198:
1177:
1127:
1126:
1125:
1122:
1111:
1110:
1109:
1101:
1100:
1083:
1082:
1081:
1078:
1007:cycloheptatriene
992:
991:
990:
982:
981:
952:Tropylium cation
913:
912:
911:
908:
887:
886:
885:
882:
837:
836:
835:
828:
827:
819:
818:
804:
802:
801:
787:
786:
785:
767:
766:
765:
739:
738:
737:
734:
723:
697:hyperconjugation
690:
689:
688:
685:
678:
677:
676:
673:
662:
650:
638:
598:
582:
574:
522:
521:
520:
517:
509:
508:
507:
504:
492:
491:
490:
487:
475:
474:
473:
470:
458:
457:
456:
453:
445:
444:
443:
440:
425:
424:
423:
420:
408:
407:
406:
403:
346:
345:
344:
341:
326:
325:
324:
321:
305:
304:
303:
300:
285:
280:
263:
262:
261:
258:
243:
223:
222:
221:
218:
198:
194:
159:As electrophiles
137:
136:
135:
132:
41:chemical species
2794:
2793:
2789:
2788:
2787:
2785:
2784:
2783:
2759:
2758:
2757:
2756:
2749:
2725:
2721:
2698:
2694:
2663:
2659:
2644:
2630:
2626:
2599:
2595:
2582:
2578:
2567:(7) 1796–1804;
2556:
2552:
2540:
2536:
2514:
2510:
2479:Olah, George A.
2476:
2472:
2457:
2453:
2442:(5), 2777–2812
2433:
2429:
2409:
2405:
2385:
2381:
2376:Wayback Machine
2354:
2350:
2325:
2321:
2306:
2292:
2288:
2273:
2259:
2255:
2220:
2216:
2196:
2192:
2160:
2156:
2124:
2120:
2105:
2091:
2087:
2072:
2056:
2052:
2037:
2023:
2019:
1987:
1983:
1968:
1954:
1950:
1935:
1921:
1917:
1910:
1896:
1892:
1872:
1868:
1862:
1842:
1838:
1823:
1809:
1805:
1794:
1788:
1773:
1759:
1755:
1750:
1746:
1739:
1725:
1721:
1691:
1687:
1683:
1679:
1674:
1670:
1665:
1648:
1580:
1558:
1547:
1546:
1545:
1535:
1531:
1507:
1503:
1498:
1494:
1485:
1474:
1473:
1472:
1462:
1458:
1449:
1436:
1432:
1427:
1420:
1419:
1408:
1404:
1402:
1399:
1398:
1382:
1379:
1378:
1377:
1375:
1370:
1367:
1366:
1365:
1363:
1353:
1349:
1343:
1338:
1334:
1330:
1326:
1310:carbon monoxide
1297:
1294:
1293:
1292:
1290:
1288:
1260:
1257:
1256:
1255:
1248:
1233:
1229:
1225:
1221:
1206:
1203:
1202:
1201:
1197:
1194:
1193:
1192:
1190:
1151:
1145:
1123:
1120:
1119:
1117:
1113:
1108:
1105:
1104:
1103:
1099:
1096:
1095:
1094:
1092:
1079:
1076:
1075:
1073:
1069:
989:
986:
985:
984:
980:
977:
976:
975:
973:
954:
948:
909:
906:
905:
903:
899:
895:
883:
880:
879:
877:
873:
856:
834:
832:
831:
830:
826:
823:
822:
821:
817:
814:
813:
812:
810:
800:
797:
796:
795:
793:
784:
781:
780:
779:
777:
772:, a mixture of
764:
762:
761:
760:
758:
735:
732:
731:
729:
725:
722:
718:
714:
702:
686:
683:
682:
680:
674:
671:
670:
668:
664:
660:
656:
652:
648:
644:
640:
636:
621:
596:
592:
588:
584:
580:
576:
572:
568:
564:
518:
515:
514:
512:
505:
502:
501:
499:
495:
488:
485:
484:
482:
478:
471:
468:
467:
465:
461:
454:
451:
450:
448:
441:
438:
437:
435:
428:
421:
418:
417:
415:
411:
404:
401:
400:
398:
394:
387:
342:
339:
338:
336:
329:
322:
319:
318:
316:
312:
308:
301:
298:
297:
295:
288:
283:
278:
274:
270:
266:
259:
256:
255:
253:
246:
241:
237:
233:
229:
219:
216:
215:
213:
206:
190:
185:
161:
133:
130:
129:
127:
123:
104:
88:
83:
77:
70:
66:
58:
17:
12:
11:
5:
2792:
2782:
2781:
2776:
2771:
2755:
2754:
2748:978-3527306732
2747:
2719:
2692:
2657:
2642:
2624:
2593:
2576:
2550:
2534:
2508:
2489:(3): 808–820.
2470:
2451:
2427:
2403:
2379:
2348:
2319:
2304:
2286:
2271:
2253:
2214:
2190:
2171:(4): 631–635.
2154:
2118:
2103:
2085:
2070:
2050:
2035:
2017:
1981:
1966:
1948:
1933:
1915:
1909:978-1891389313
1908:
1890:
1866:
1860:
1836:
1821:
1803:
1786:
1771:
1753:
1744:
1737:
1719:
1700:(1): 109–116.
1689:
1685:
1681:
1677:
1667:
1666:
1664:
1661:
1660:
1659:
1654:
1647:
1644:
1640:Koch reactions
1579:
1576:
1575:
1574:
1561:
1554:
1551:
1544:
1538:
1530:
1526:
1515:
1510:
1501:
1493:
1488:
1481:
1478:
1471:
1465:
1457:
1452:
1447:
1439:
1430:
1423:
1418:
1411:
1380:
1368:
1351:
1341:
1336:
1325:
1322:
1302:
1301:
1295:
1286:
1247:
1244:
1231:
1227:
1223:
1219:
1204:
1195:
1179:
1178:
1147:Main article:
1144:
1141:
1115:
1106:
1097:
1071:
1059:Eggers Doering
987:
978:
950:Main article:
947:
944:
901:
897:
875:
855:
852:
847:carbenium ions
833:
824:
815:
798:
782:
763:
753:by removing a
747:
746:
727:
720:
716:
711:
704:
700:
666:
658:
654:
646:
642:
620:
617:
605:crystal violet
594:
590:
586:
578:
570:
566:
558:
557:
554:
551:
548:
545:
542:
539:
536:
533:
531:HIA (kcal/mol)
527:
526:
524:(least stable)
510:
497:
493:
480:
476:
463:
459:
446:
433:
426:
413:
409:
396:
392:
385:
379:
378:
375:
372:
369:
366:
363:
360:
357:
354:
352:HIA (kcal/mol)
348:
347:
334:
327:
314:
310:
306:
293:
286:
281:
276:
272:
268:
264:
251:
244:
239:
235:
231:
227:
211:
204:
189:
186:
184:
181:
160:
157:
125:
113:rate constants
103:
102:Rearrangements
100:
87:
84:
79:Main article:
76:
73:
68:
64:
56:
53:carbonium ions
15:
9:
6:
4:
3:
2:
2791:
2780:
2777:
2775:
2772:
2770:
2767:
2766:
2764:
2750:
2744:
2740:
2736:
2732:
2731:
2723:
2715:
2711:
2708:(1–2): 1–17.
2707:
2703:
2696:
2688:
2684:
2680:
2676:
2672:
2668:
2661:
2653:
2649:
2645:
2643:9780387448978
2639:
2635:
2628:
2620:
2616:
2612:
2608:
2604:
2597:
2591:
2587:
2586:
2580:
2574:
2570:
2566:
2562:
2561:
2554:
2547:
2543:
2538:
2532:
2528:
2525:(4) 900–908;
2524:
2520:
2519:
2512:
2504:
2500:
2496:
2492:
2488:
2484:
2480:
2474:
2468:
2466:(4), 984–987
2465:
2461:
2455:
2449:
2445:
2441:
2437:
2431:
2425:
2421:
2417:
2413:
2407:
2401:
2397:
2394:: 3108–3126.
2393:
2389:
2383:
2377:
2373:
2370:
2366:
2362:
2358:
2352:
2346:
2342:
2338:
2334:
2333:
2328:
2323:
2315:
2311:
2307:
2305:9780387448978
2301:
2297:
2290:
2282:
2278:
2274:
2272:9780387448978
2268:
2264:
2257:
2249:
2245:
2241:
2237:
2233:
2229:
2225:
2218:
2212:
2208:
2204:
2201:
2194:
2186:
2182:
2178:
2174:
2170:
2166:
2158:
2150:
2146:
2142:
2138:
2134:
2130:
2122:
2114:
2110:
2106:
2104:9780387448978
2100:
2096:
2089:
2081:
2077:
2073:
2067:
2063:
2062:
2054:
2046:
2042:
2038:
2036:9780387448978
2032:
2028:
2021:
2013:
2009:
2005:
2001:
1997:
1993:
1985:
1977:
1973:
1969:
1967:9780470276105
1963:
1959:
1952:
1944:
1940:
1936:
1934:9780387448978
1930:
1926:
1919:
1911:
1905:
1901:
1894:
1888:
1884:
1880:
1876:
1870:
1863:
1857:
1853:
1852:
1847:
1840:
1832:
1828:
1824:
1822:9780387448978
1818:
1814:
1807:
1800:
1790:
1782:
1778:
1774:
1772:9780387448978
1768:
1764:
1757:
1748:
1740:
1738:0-06-044083-X
1734:
1730:
1723:
1715:
1711:
1707:
1703:
1699:
1695:
1684:] [Al
1672:
1668:
1658:
1657:Nitrenium ion
1655:
1653:
1650:
1649:
1643:
1641:
1637:
1632:
1630:
1626:
1621:
1619:
1615:
1611:
1610:alpha-olefins
1607:
1602:
1600:
1596:
1588:
1584:
1559:
1549:
1542:
1536:
1528:
1524:
1513:
1508:
1499:
1486:
1476:
1469:
1463:
1455:
1450:
1437:
1428:
1409:
1397:
1396:
1395:
1393:
1389:
1360:
1358:
1357:aryldiazonium
1346:
1321:
1319:
1315:
1311:
1307:
1284:
1283:
1282:
1280:
1276:
1272:
1268:
1253:
1243:
1241:
1238:deduced from
1237:
1217:
1213:
1212:benzenium ion
1188:
1183:
1176:
1172:
1171:
1170:
1168:
1164:
1160:
1156:
1150:
1137:
1133:
1129:
1090:
1085:
1067:
1062:
1060:
1055:
1053:
1048:
1046:
1043:
1039:
1035:
1034:Hückel's rule
1031:
1027:
1023:
1018:
1016:
1012:
1008:
1004:
1000:
996:
971:
967:
966:tropylium ion
958:
953:
938:
934:
931:
927:
923:
921:
917:
893:
892:
871:
870:
865:
861:
848:
843:
839:
808:
791:
775:
771:
756:
752:
744:
712:
709:
705:
698:
694:
693:
692:
630:
625:
619:Alkylium ions
616:
614:
610:
606:
602:
555:
552:
549:
546:
543:
540:
537:
534:
532:
529:
528:
525:
511:
494:
477:
460:
447:
431:
427:
410:
390:
386:
384:
381:
380:
376:
373:
370:
367:
364:
361:
358:
355:
353:
350:
349:
332:
328:
307:
291:
287:
282:
265:
249:
245:
228:
226:
225:(most stable)
209:
205:
203:
200:
199:
193:
176:
172:
170:
166:
156:
154:
150:
149:Wolff–Kishner
147:(followed by
146:
142:
121:
120:
114:
109:
99:
97:
93:
82:
72:
62:
54:
50:
46:
45:formal charge
42:
38:
34:
33:carbenium ion
26:
21:
16:Class of ions
2779:Carbocations
2728:
2722:
2705:
2701:
2695:
2670:
2666:
2660:
2633:
2627:
2610:
2606:
2602:
2596:
2583:
2579:
2564:
2558:
2553:
2548:; pp 130–133
2541:
2537:
2522:
2516:
2511:
2486:
2482:
2473:
2463:
2459:
2454:
2439:
2435:
2430:
2415:
2411:
2406:
2391:
2387:
2382:
2364:
2360:
2356:
2351:
2330:
2322:
2295:
2289:
2262:
2256:
2231:
2227:
2223:
2217:
2202:
2199:
2193:
2168:
2164:
2157:
2132:
2128:
2121:
2094:
2088:
2060:
2053:
2026:
2020:
1995:
1991:
1984:
1957:
1951:
1924:
1918:
1899:
1893:
1878:
1874:
1869:
1850:
1846:March, Jerry
1839:
1812:
1806:
1789:
1762:
1756:
1747:
1728:
1722:
1697:
1693:
1671:
1652:Borenium ion
1633:
1622:
1603:
1592:
1361:
1347:
1327:
1314:mass spectra
1303:
1285:RCOCl + SbCl
1279:acyl halides
1273:such as the
1249:
1246:Acylium ions
1236:Bond lengths
1211:
1184:
1180:
1166:
1162:
1154:
1152:
1143:Arenium ions
1130:
1086:
1066:antiaromatic
1063:
1056:
1049:
1029:
1025:
1019:
965:
963:
926:Oxocarbenium
924:
919:
889:
867:
857:
846:
748:
707:
634:
628:
561:
530:
523:
429:
388:
382:
351:
330:
289:
247:
224:
207:
201:
191:
165:nucleophiles
162:
116:
105:
89:
81:carbocations
75:Nomenclature
63:such as B(CH
49:carbocations
37:positive ion
32:
30:
24:
2590:acyl groups
2546:Peter Sykes
1881:, 287–344.
1252:acylium ion
1155:arenium ion
1149:Arenium ion
719:OH·SbF
613:heteroatoms
383:Carbocation
284:2-norbornyl
202:Carbocation
2763:Categories
2436:Chem. Rev.
2071:0060440848
1663:References
1618:detergents
1606:alkylation
1388:beta decay
1333:, IPh, or
1022:heptagonal
864:heteroatom
770:magic acid
169:superacids
108:rearranges
86:Reactivity
2687:0002-7863
2652:154040953
2503:0002-7863
2314:154040953
2281:154040953
2185:0022-3263
2149:0002-7863
2113:154040953
2045:154040953
2012:0002-7863
1976:286483846
1943:154040953
1831:154040953
1799:help page
1781:154040953
1553:¯
1550:ν
1537:−
1500:≡
1492:⟶
1480:¯
1477:ν
1464:−
1429:≡
1417:⟶
1410:≡
1306:ångströms
1167:σ-complex
860:resonance
807:isobutane
805:), turns
188:Stability
145:acylation
96:tropylium
2563:; 2003;
2521:; 1942;
2462:, 1970,
2438:, 2004,
2414:, 1954,
2372:Archived
2337:molecule
2248:15170300
2080:14214254
1848:(2007),
1714:23180742
1692:]".
1646:See also
1627:are the
1612:to give
1587:Fuchsine
999:atropine
970:aromatic
2769:Cations
1392:tritium
1318:ketones
1187:benzene
1165:, or a
1011:bromine
995:tropine
930:iminium
894:cation
872:cation
757:anion,
755:hydride
751:alkanes
741:group (
449:CH≡C−CH
61:boranes
2745:
2685:
2650:
2640:
2501:
2312:
2302:
2279:
2269:
2246:
2183:
2147:
2111:
2101:
2078:
2068:
2043:
2033:
2010:
1974:
1964:
1941:
1931:
1906:
1858:
1829:
1819:
1779:
1769:
1735:
1712:
1218:, H(CB
1210:. The
1038:ligand
968:is an
891:benzyl
878:=CH−CH
788:) and
679:, and
601:trityl
416:=CH−CH
2359:Vol.
2327:IUPAC
1751:March
1289:→ RCO
1045:atoms
1042:metal
869:allyl
701:CH/CC
92:octet
35:is a
2743:ISBN
2683:ISSN
2648:OCLC
2638:ISBN
2499:ISSN
2369:link
2310:OCLC
2300:ISBN
2277:OCLC
2267:ISBN
2244:PMID
2224:tert
2181:ISSN
2145:ISSN
2109:OCLC
2099:ISBN
2076:OCLC
2066:ISBN
2041:OCLC
2031:ISBN
2008:ISSN
1972:OCLC
1962:ISBN
1939:OCLC
1929:ISBN
1904:ISBN
1856:ISBN
1827:OCLC
1817:ISBN
1777:OCLC
1767:ISBN
1733:ISBN
1710:PMID
1638:and
1604:The
1291:SbCl
1222:H(CH
964:The
928:and
888:and
556:312
553:298
550:287
547:273
544:270
541:258
538:256
535:249
377:246
374:234
371:231
368:231
365:222
362:221
359:215
356:201
117:see
25:tert
23:The
2735:doi
2710:doi
2706:398
2675:doi
2671:110
2615:doi
2569:doi
2565:125
2527:doi
2491:doi
2444:doi
2440:104
2420:doi
2396:doi
2341:doi
2339:".
2236:doi
2207:doi
2173:doi
2137:doi
2133:106
2000:doi
1883:doi
1879:163
1702:doi
1390:of
1355:in
1331:TfO
1316:of
1250:An
1153:An
1040:to
1013:or
904:−CH
811:(CH
794:FSO
778:SbF
715:FSO
653:(CH
641:(CH
565:(CH
151:or
2765::
2741:.
2704:.
2681:.
2669:.
2646:.
2611:94
2609:.
2588:,
2544:,
2523:64
2497:.
2487:94
2485:.
2464:92
2416:76
2392:24
2390:,
2365:43
2329:,
2308:.
2275:.
2242:.
2232:43
2230:.
2203:89
2179:.
2169:49
2167:.
2143:.
2131:.
2107:.
2074:.
2039:.
2006:.
1996:89
1994:.
1970:.
1937:.
1877:,
1825:.
1801:).
1795::0
1775:.
1708:.
1698:19
1696:.
1688:Br
1642:.
1631:.
1620:.
1518:He
1496:RC
1443:He
1425:RC
1414:CT
1406:RC
1394::
1376:CH
1364:CH
1320:.
1281::
1230:Br
1220:11
1189:,
1169:.
1091:,
1047:.
1017:.
874:CH
838:.
681:CH
669:CH
665:CH
663:,
661:CH
651:,
585:(C
513:CH
432:-C
412:CH
399:CH
391:-C
333:-C
317:CH
292:-C
279:CH
267:(C
250:-C
230:(C
210:-C
171:.
134:11
71:.
31:A
2751:.
2737::
2716:.
2712::
2689:.
2677::
2654:.
2621:.
2617::
2603:p
2571::
2529::
2505:.
2493::
2446::
2422::
2398::
2361:5
2343::
2316:.
2283:.
2250:.
2238::
2209::
2187:.
2175::
2151:.
2139::
2115:.
2082:.
2047:.
2014:.
2002::
1978:.
1945:.
1912:.
1885::
1833:.
1783:.
1741:.
1716:.
1704::
1690:7
1686:2
1682:3
1680:)
1678:3
1560:e
1543:+
1533:e
1529:+
1525:3
1514:+
1509:+
1505:C
1487:e
1470:+
1460:e
1456:+
1451:+
1446:]
1438:3
1434:C
1422:[
1381:3
1369:3
1352:2
1350:N
1342:N
1337:2
1335:N
1296:6
1287:5
1261:C
1258:+
1232:6
1228:5
1226:)
1224:3
1205:7
1200:H
1196:6
1191:C
1124:3
1121:+
1118:H
1116:3
1114:C
1107:3
1102:H
1098:3
1093:C
1080:5
1077:+
1074:H
1072:5
1070:C
1030:n
1026:n
988:7
983:H
979:7
974:C
910:2
907:+
902:5
900:H
898:6
896:C
884:2
881:+
876:2
829:C
825:3
820:)
816:3
803:H
799:3
792:(
783:5
776:(
759:H
736:5
733:+
730:H
728:2
726:C
721:5
717:2
708:s
687:3
684:+
675:2
672:+
667:3
659:2
657:)
655:3
649:C
647:3
645:)
643:3
629:t
597:C
595:3
593:)
591:5
589:H
587:5
581:C
579:3
577:H
573:C
571:3
569:)
567:3
519:3
516:+
506:5
503:+
500:H
498:6
496:C
489:3
486:+
483:H
481:2
479:C
472:5
469:+
466:H
464:2
462:C
455:2
452:+
442:5
439:+
436:H
434:5
430:c
422:2
419:+
414:2
405:2
402:+
397:5
395:H
393:3
389:c
343:7
340:+
337:H
335:3
331:i
323:2
320:+
315:5
313:H
311:6
309:C
302:9
299:+
296:H
294:4
290:t
277:2
275:)
273:5
271:H
269:6
260:3
257:+
254:H
252:3
248:c
242:C
240:3
238:)
236:5
234:H
232:6
220:7
217:+
214:H
212:7
208:c
131:+
128:H
126:6
124:C
69:3
67:)
65:3
57:5
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.