69:
47:
213:
223:
One drawback of the Zincke
Aldehyde synthesis is the need for 2 equivalents of the amine in the initial pyridine ring opening reaction. This is of particular concern for the case of complex secondary amines required for natural product synthesis. The group of Marazano recently found an alternative
111:
123:
139:
175:
783:
Nguyen, T.; Peixoto, S.; Ouairy, C.; Nguyen, T.; Bénéchie, M.; Marazano, C.; Michel, P. (2010). "Simple and
Convenient Method for the Synthesis of 2-Substituted Glutaconaldehyde Salts and 2-Substituted Glutaconaldehyde Derivatives".
183:
130:
The
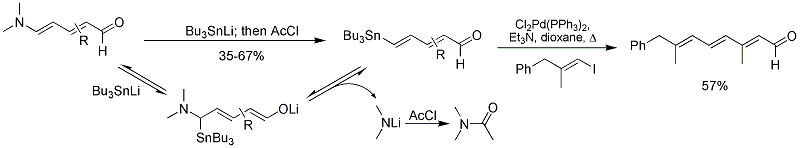
Vanderwal group has also reported the synthesis of 4-stannyldienals from Zincke aldehydes by addition of tributylstannyl anion and quenching with acetyl chloride. The products are useful substrates for
212:
23:, are the product of the reaction of a pyridinium salt with two equivalents of any secondary amine, followed by basic hydrolysis. Using secondary amines (as opposed to primary amines) the
671:
Martin, D. B. C.; Vanderwal, C. D. (2009). "Efficient Access to the Core of the
Strychnos, Aspidosperma and Iboga Alkaloids. A Short Synthesis of Norfluorocurarine".
735:
Nuhant, P.; Raikar, S. B.; Wypych, J.-C.; Delpech, B.; Marazano, C. (2009). "Enhancement of 5-Aminopenta-2,4-dienals
Electrophilicity via Activation by
150:
reported another interesting rearrangement of Zincke aldehydes. Tryptamine-derived Zincke aldehydes are heated with strong base to give the rearranged
110:
122:
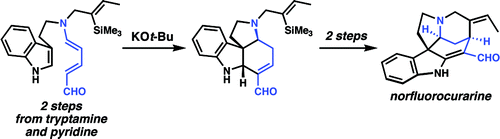
167:, becoming the shortest synthesis of strychnine reported to date at only six linear steps. This works has been highlighted on the blog
138:
483:
Kearney, Aaron M.; Vanderwal, Christopher D. (2006). "Synthesis of
Nitrogen Heterocycles by the Ring Opening of Pyridinium Salts".
96:
88:
174:
559:
Steinhardt, S. E.; Vanderwal, C. D. (2009). "Complex
Polycyclic Lactams from Pericyclic Cascade Reactions of Zincke Aldehydes".
103:
revealed an unusual and unexpected mechanism based on computational studies. The new mechanism involves formation of a vinyl
635:
Michels, T.; Rhee, J. U.; Vanderwal, C. D. (2008). "Synthesis of δ-Tributylstannyl
Unsaturated Aldehydes from Pyridines".
228:
derivatives using TFA. This solution has greatly simplified the production and purification of complex Zincke aldehydes.
68:
328:
290:
254:
219:-Acyl Pictet−Spengler Reaction by Treatment of Tryptamine and Homoveratrylamine Derived Aminopentadienals with TFAA
95:-product stereospecifically. In a follow-up paper, allylic amines were used and gave products of a rearrangement /
707:
Martin, D. B. C.; Vanderwal, C. D. (2011). "A Synthesis of
Strychnine by a Longest Linear Sequence of Six Steps".
27:
takes on a different shape forming Zincke aldehydes in which the pyridine ring is ring-opened with the terminal
182:
209:
natural products, and was applied to the construction of a known intermediate in a previous total synthesis.
422:"Derivatives and reactions of glutaconaldehyde—XIII Regiospecific ring opening of 3-substituted pyridines"
846:
99:
cascade. Mechanistic details were also discussed, however further investigations in collaboration with
595:
Paton, R. S.; Steinhardt, S. E.; Vanderwal, C. D.; Houk, K. N. (2011). "Stereocontrolled
Synthesis of
191:
457:
154:
as shown below. This reaction was the key step in their total synthesis of norfluorocurarine, a
391:
Becher, J. (1980). "Synthesis and Reactions of Glutaconaldehyde and 5-Amino-2,4-pentadienals".
202:
519:
Steinhardt, S. E.; Silverston, J. S.; Vanderwal, C. D. (2008). "Stereocontrolled Synthesis of
485:
426:
46:
8:
786:
599:-Dienes via an Unexpected Pericyclic Cascade Rearrangement of 5-Amino-2,4-pentadienals".
523:-Dienes via an Unexpected Pericyclic Cascade Rearrangement of 5-Amino-2,4-pentadienals".
393:
594:
841:
440:
421:
851:
765:
689:
673:
653:
617:
601:
577:
561:
541:
525:
501:
194:
appeared from the group of Christian Marazano. This reaction provided the tetrahydro-
795:
757:
717:
681:
645:
609:
569:
533:
493:
465:
435:
402:
373:
337:
299:
263:
225:
77:
40:
35:. The use of the dinitrophenyl group for pyridine activation was first reported by
518:
364:
132:
58:
24:
816:
147:
734:
319:
281:
245:
36:
469:
835:
749:
377:
341:
304:
285:
267:
195:
799:
769:
693:
657:
621:
581:
545:
505:
497:
168:
782:
406:
87:-unsaturated amides was discovered serendipitously while trying to do an
324:"Über Dinitrophenylisochinoliniumchlorid und dessen Umwandlungsprodukte"
721:
709:
637:
164:
761:
685:
649:
634:
613:
573:
537:
286:"Ueber Dinitrophenylpyridiniumchlorid und dessen Umwandlungsproducte"
250:"Ueber Dinitrophenylpyridiniumchlorid und dessen Umwandlungsproducte"
156:
455:
Cheng, W.-C.; Kurth, M. J. (2002). "The Zincke Reaction: A Review".
359:
323:
249:
826:
419:
206:
160:
83:
More recently, an interesting rearrangement of Zincke aldehydes to
32:
558:
360:"Über eine neue, vom Pyridin derivierende Klasse von Farbstoffen"
190:
Also in 2009, the first reports of Zincke aldehydes undergoing a
54:
The synthesis and utility of Zincke aldehydes has been reviewed.
28:
821:
706:
670:
126:
New ketene-based mechanism for rearrangement of Zincke aldehydes
100:
43:
for pyridine activation was independently reported by W. König:
280:
244:
104:
62:
318:
151:
163:. This strategy was also employed in a short synthesis of
454:
135:reactions to give interesting polyene structures.
142:Formation of stannyldienals from Zincke aldehydes
833:
482:
420:Becher, J.; Finsen, L.; Winckelmann, I. (1981).
80:mediated pyridine activation (König method).
743:-Bistrifluoroacetylation. Application to an
224:synthesis by condensation onto a variety of
61:has been applied in the synthesis of novel
390:
439:
357:
303:
353:
351:
178:Formal cycloaddition of Zincke aldehydes
834:
348:
114:Rearrangement of Zincke aldehydes to
89:intramolecular Diels-Alder reaction
13:
211:
181:
173:
137:
121:
109:
67:
45:
14:
863:
810:
747:-Acyl Pictet−Spengler Reaction".
329:Justus Liebigs Annalen der Chemie
291:Justus Liebigs Annalen der Chemie
284:; Heuser, G.; Moller, W. (1904).
255:Justus Liebigs Annalen der Chemie
248:; Heuser, G.; Moller, W. (1904).
186:Vanderwal synthesis of strychnine
776:
728:
700:
664:
628:
588:
552:
512:
476:
448:
413:
384:
312:
274:
238:
91:. The rearrangement gives the
1:
441:10.1016/S0040-4020(01)88892-X
231:
72:Zincke aldehydes Kearney 2006
322:; Weisspfenning, G. (1913).
7:
10:
868:
97:intramolecular Diels-Alder
470:10.1080/00304940209355784
817:Vanderwal Group Homepage
378:10.1002/prac.19040690107
342:10.1002/jlac.19133960107
305:10.1002/jlac.19043300217
268:10.1002/jlac.19043330212
192:Pictet-Spengler reaction
21:5-aminopenta-2,4-dienals
458:Org. Prep. Proced. Int.
31:group hydrolyzed to an
827:Totally Synthetic blog
800:10.1055/s-0029-1217105
498:10.1002/anie.200602996
220:
203:tetrahydroisoquinoline
187:
179:
143:
127:
119:
73:
51:
486:Angew. Chem. Int. Ed.
215:
205:core present in many
185:
177:
141:
133:Stille cross-coupling
125:
113:
71:
49:
407:10.1055/s-1980-29134
822:Houk Group Homepage
148:the Vanderwal group
118:-unsaturated amides
57:A variation of the
847:Alkene derivatives
722:10.1039/C1SC00009H
358:König, W. (1904).
221:
188:
180:
144:
128:
120:
74:
52:
762:10.1021/jo9019545
686:10.1021/ja900640v
680:(10): 3472–3473.
674:J. Am. Chem. Soc.
650:10.1021/ol8020435
644:(21): 4787–4790.
614:10.1021/ja107988b
608:(11): 3895–3905.
602:J. Am. Chem. Soc.
574:10.1021/ja902439f
568:(22): 7546–7547.
562:J. Am. Chem. Soc.
538:10.1021/ja8028125
532:(24): 7560–7561.
526:J. Am. Chem. Soc.
492:(46): 7803–7806.
434:(13): 2375–2378.
169:Totally Synthetic
859:
804:
803:
780:
774:
773:
732:
726:
725:
704:
698:
697:
668:
662:
661:
632:
626:
625:
592:
586:
585:
556:
550:
549:
516:
510:
509:
480:
474:
473:
452:
446:
445:
443:
417:
411:
410:
388:
382:
381:
355:
346:
345:
316:
310:
309:
307:
278:
272:
271:
262:(2–3): 296–345.
242:
226:glutaconaldehyde
78:cyanogen bromide
50:Zincke aldehydes
41:cyanogen bromide
17:Zincke aldehydes
867:
866:
862:
861:
860:
858:
857:
856:
832:
831:
813:
808:
807:
781:
777:
756:(24): 9413–21.
733:
729:
705:
701:
669:
665:
633:
629:
593:
589:
557:
553:
517:
513:
481:
477:
453:
449:
418:
414:
389:
385:
365:J. Prakt. Chem.
356:
349:
317:
313:
279:
275:
243:
239:
234:
59:Zincke reaction
25:Zincke reaction
12:
11:
5:
865:
855:
854:
849:
844:
830:
829:
824:
819:
812:
811:External links
809:
806:
805:
794:(1): 103–109.
775:
727:
699:
663:
627:
587:
551:
511:
475:
464:(6): 587–608.
447:
412:
401:(8): 589–612.
383:
372:(1): 105–137.
347:
336:(1): 103–131.
311:
298:(2): 361–374.
273:
236:
235:
233:
230:
101:the Houk group
37:Theodor Zincke
9:
6:
4:
3:
2:
864:
853:
850:
848:
845:
843:
840:
839:
837:
828:
825:
823:
820:
818:
815:
814:
801:
797:
793:
789:
788:
779:
771:
767:
763:
759:
755:
752:
751:
750:J. Org. Chem.
746:
742:
738:
731:
723:
719:
715:
712:
711:
703:
695:
691:
687:
683:
679:
676:
675:
667:
659:
655:
651:
647:
643:
640:
639:
631:
623:
619:
615:
611:
607:
604:
603:
598:
591:
583:
579:
575:
571:
567:
564:
563:
555:
547:
543:
539:
535:
531:
528:
527:
522:
515:
507:
503:
499:
495:
491:
488:
487:
479:
471:
467:
463:
460:
459:
451:
442:
437:
433:
429:
428:
423:
416:
408:
404:
400:
396:
395:
387:
379:
375:
371:
368:(in German).
367:
366:
361:
354:
352:
343:
339:
335:
332:(in German).
331:
330:
325:
321:
315:
306:
301:
297:
294:(in German).
293:
292:
287:
283:
277:
269:
265:
261:
258:(in German).
257:
256:
251:
247:
241:
237:
229:
227:
218:
214:
210:
208:
204:
200:
198:
193:
184:
176:
172:
170:
166:
162:
159:
158:
153:
149:
140:
136:
134:
124:
117:
112:
108:
106:
102:
98:
94:
90:
86:
81:
79:
70:
66:
64:
60:
55:
48:
44:
42:
39:. The use of
38:
34:
30:
26:
22:
18:
791:
785:
778:
753:
748:
744:
740:
736:
730:
713:
708:
702:
677:
672:
666:
641:
636:
630:
605:
600:
596:
590:
565:
560:
554:
529:
524:
520:
514:
489:
484:
478:
461:
456:
450:
431:
425:
415:
398:
392:
386:
369:
363:
333:
327:
314:
295:
289:
276:
259:
253:
240:
222:
216:
196:
189:
155:
145:
129:
115:
92:
84:
82:
75:
56:
53:
20:
16:
15:
427:Tetrahedron
320:Zincke, Th.
282:Zincke, Th.
246:Zincke, Th.
836:Categories
716:(4): 649.
710:Chem. Sci.
638:Org. Lett.
232:References
199:-carboline
165:strychnine
842:Aldehydes
787:Synthesis
394:Synthesis
157:Strychnos
146:In 2009,
852:Enamines
770:19924881
694:19236094
658:18817407
622:21351736
582:19449870
546:18505251
506:17072923
207:alkaloid
161:alkaloid
33:aldehyde
63:indoles
29:iminium
768:
692:
656:
620:
580:
544:
504:
105:ketene
76:with
19:, or
792:2010
766:PMID
690:PMID
654:PMID
618:PMID
578:PMID
542:PMID
502:PMID
399:1980
152:enal
796:doi
758:doi
718:doi
682:doi
678:131
646:doi
610:doi
606:133
570:doi
566:131
534:doi
530:130
494:doi
466:doi
436:doi
403:doi
374:doi
338:doi
334:396
300:doi
296:330
264:doi
260:333
201:or
838::
790:.
764:.
754:74
688:.
652:.
642:10
616:.
576:.
540:.
500:.
490:45
462:34
432:37
430:.
424:.
397:.
370:69
362:.
350:^
326:.
288:.
252:.
171:.
107:.
65::
802:.
798::
772:.
760::
745:N
741:N
739:,
737:O
724:.
720::
714:2
696:.
684::
660:.
648::
624:.
612::
597:Z
584:.
572::
548:.
536::
521:Z
508:.
496::
472:.
468::
444:.
438::
409:.
405::
380:.
376::
344:.
340::
308:.
302::
270:.
266::
217:N
197:β
116:Z
93:Z
85:Z
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.