194:
324:
75:
2618:
492:, temperature-dependent powder diffraction. Increased knowledge of the phase relations often leads to further refinement in synthetic procedures in an iterative way. New phases are thus characterized by their melting points and their stoichiometric domains. The latter is important for the many solids that are non-stoichiometric compounds. The cell parameters obtained from XRD are particularly helpful to characterize the homogeneity ranges of the latter.
2642:
444:
412:
2654:
172:
2630:
1676:
456:
be coupled to achieve a better effect. For example, SEM is a useful complement to EDX due to its focused electron beam, it produces a high-magnification image that provides information on the surface topography. Once the area of interest has been identified, EDX can be used to determine the elements present in that specific spot.
66:, make solid-state materials. Solids can be classified as crystalline or amorphous on basis of the nature of order present in the arrangement of their constituent particles. Their elemental compositions, microstructures, and physical properties can be characterized through a variety of analytical methods.
455:
analysis (XRD) involves the generation of characteristic X-rays upon interaction with the sample. The intensity of diffracted rays scattered at different angles is used to analyze the physical properties of a material such as phase composition and crystallographic structure. These techniques can also
419:
Once the unit cell of a new phase is known, the next step is to establish the stoichiometry of the phase. This can be done in several ways. Sometimes the composition of the original mixture will give a clue, under the circumstances that only a product with a single powder pattern is found or a phase
402:
because many solid-state reactions will produce polycrystalline molds or powders. Powder diffraction aids in the identification of known phases in the mixture. If a pattern is found that is not known in the diffraction data libraries, an attempt can be made to index the pattern. The characterization
205:
Crucible materials have a great role to play in molten flux synthesis. The crucible should not react with the flux or the starting reagent. If any of the material is volatile, it is recommended to conduct the reaction in a sealed ampule. If the target phase is sensitive to oxygen, a carbon- coated
201:
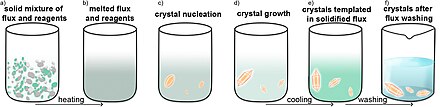
Molten flux synthesis can be an efficient method for obtaining single crystals. In this method, the starting reagents are combined with flux, an inert material with a melting point lower than that of the starting materials. The flux serves as a solvent. After the reaction, the excess flux can be
136:
The ceramic method is one of the most common synthesis techniques. The synthesis occurs entirely in the solid state. The reactants are ground together, formed into a pellet using a pellet press and hydraulic press, and heated at high temperatures. When the temperature of the reactants are
440:(EDX) is a technique that uses electron beam excitation. Exciting the inner shell of an atom with incident electrons emits characteristic X-rays with specific energy to each element. The peak energy can identify the chemical composition of a sample, including the distribution and concentration.
85:
Because of its direct relevance to products of commerce, solid state inorganic chemistry has been strongly driven by technology. Progress in the field has often been fueled by the demands of industry, sometimes in collaboration with academia. Applications discovered in the 20th century include
398:, a numerical relationship between the quantities of reactant and product, is typically varied systematically. It is important to find which stoichiometries will lead to new solid compounds or solid solutions between known ones. A prime method to characterize the reaction products is
532:
For metallic materials, their optical properties arise from the collective excitation of conduction electrons. The coherent oscillations of electrons under electromagnetic radiation along with associated oscillations of the electromagnetic field are called
217:
results in very pure materials. The reaction typically occurs in a sealed ampoule. A transporting agent, added to the sealed ampoule, produces a volatile intermediate species from the solid reactant. For metal oxides, the transporting agent is usually
160:. A chemist forms pellets from the ground reactants and places the pellets into containers for heating. The choice of container depends on the precursors, the reaction temperature and the expected product. For example,
222:
or HCl. The ampoule has a temperature gradient, and, as the gaseous reactant travels along the gradient, it eventually deposits as a crystal. An example of an industrially-used chemical vapor transport reaction is the
118:
Given the diversity of solid-state compounds, an equally diverse array of methods are used for their preparation. Synthesis can range from high-temperature methods, like the ceramic method, to gas methods, like
94:-based catalysts for petroleum processing in the 1950s, high-purity silicon as a core component of microelectronic devices in the 1960s, and “high temperature” superconductivity in the 1980s. The invention of
423:
Often, considerable effort in refining the synthetic procedures is required to obtain a pure sample of the new material. If it is possible to separate the product from the rest of the reaction mixture,
608:
Kanatzidis, Mercouri G. (2018). "Report from the third workshop on future directions of solid-state chemistry: The status of solid-state chemistry and its impact in the physical sciences".
436:(TEM) can be used. The detection of scattered and transmitted electrons from the surface of the sample provides information about the surface topography and composition of the material.
560:
In many cases, new solid compounds are further characterized by a variety of techniques that straddle the fine line that separates solid-state chemistry from solid-state physics. See
1016:
Rajapakse, Manthila; Karki, Bhupendra; Abu, Usman O.; Pishgar, Sahar; Musa, Md Rajib Khan; Riyadh, S. M. Shah; Yu, Ming; Sumanasekera, Gamini; Jasinski, Jacek B. (2021-03-10).
472:
and refining the preparative procedures and that are linked to the question of which phases are stable at what composition and what stoichiometry. In other words, what the
394:
Synthetic methodology and characterization often go hand in hand in the sense that not one but a series of reaction mixtures are prepared and subjected to heat treatment.
537:. The excitation wavelength and frequency of the plasmon resonances provide information on the particle's size, shape, composition, and local optical environment.
355:. Such reactions are conducted in open-ended tubes, which the gasses are passed through. Also, these reactions can take place inside a measuring device such as a
1686:
548:
that represents the minimum energy difference between the top of the valence band and the bottom of the conduction band. The band gap can be determined using
137:
sufficient, the ions at the grain boundaries react to form desired phases. Generally ceramic methods give polycrystalline powders, but not single crystals.
386:
This is the process in which a material’s chemical composition, structure, and physical properties are determined using a variety of analytical techniques.
967:
Laipan, Minwang; Xiang, Lichen; Yu, Jingfang; Martin, Benjamin R.; Zhu, Runliang; Zhu, Jianxi; He, Hongping; Clearfield, Abraham; Sun, Luyi (2020-04-01).
106:'s work on oxidation rate theory, counter diffusion of ions, and defect chemistry. Because of his contributions, he has sometimes been referred to as the
516:
are very sensitive to small changes caused by lattice expansion/compression (thermal or pressure), phase changes, or local defects. Common methods are
206:
fused silica tube or a carbon crucible inside a fused silica tube is often used which prevents the direct contact between the tube wall and reagents.
62:
with a focus on the synthesis of novel materials and their characterization. A diverse range of synthetic techniques, such as the ceramic method and
700:
699:
Ben Smida, Youssef; Marzouki, Riadh; Kaya, Savaş; Erkan, Sultan; Faouzi Zid, Mohamed; Hichem
Hamzaoui, Ahmed (2020-10-07), Marzouki, Riadh (ed.),
1713:
843:
1814:
102:
was an enabling innovation. Our understanding of how reactions proceed at the atomic level in the solid state was advanced considerably by
1819:
1688:, Sadoway, Donald. 3.091SC; Introduction to Solid State Chemistry, Fall 2010. (Massachusetts Institute of Technology: MIT OpenCourseWare)
202:
washed away using an appropriate solvent or it can be heat again to remove the flux by sublimation if it is a volatile compound.
1797:
1841:
1680:
1570:
1424:
1389:
1343:
1159:
819:
722:
1853:
1792:
561:
549:
437:
1706:
859:"Outstanding Advantages, Current Drawbacks, and Significant Recent Developments in Mechanochemistry: A Perspective View"
468:
X-ray diffraction is also used due to its imaging capabilities and speed of data generation. The latter often requires
1660:
1534:
1128:
1086:
592:
481:
433:
2658:
1593:"How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra"
460:
can be coupled with TEM or SEM to investigate the level of crystallinity and the lattice parameters of a sample.
1065:
Fromhold, Albert T.; Fromhold, Regina G. (1984-01-01), Bamford, C. H.; Tipper, C. F. H.; Compton, R. G. (eds.),
2680:
2537:
1699:
676:
1980:
1734:
521:
485:
1018:"Intercalation as a versatile tool for fabrication, property tuning, and phase transitions in 2D materials"
429:
2257:
1744:
214:
898:
378:
from molecular precursors. A carrier gas transports the gaseous precursors to the material for coating.
2685:
2634:
2183:
2154:
2134:
2087:
1183:
371:
356:
1151:
Handbook of deposition technologies for films and coatings : science, applications and technology
1108:
1066:
755:
251:
synthesis is the insertion of molecules or ions between layers of a solid. The layered solid has weak
1772:
534:
457:
296:
248:
120:
63:
517:
2527:
2443:
2082:
2465:
2376:
2339:
2223:
2149:
1970:
1953:
1896:
1107:
Koga, Y.; Harrison, L. G. (1984-01-01), Bamford, C. H.; Tipper, C. F. H.; Compton, R. G. (eds.),
513:
2383:
2371:
2262:
2127:
1901:
1767:
264:
99:
2532:
2429:
2414:
2344:
2267:
2049:
1958:
1883:
1782:
1441:
930:
452:
95:
30:, structure, and properties of solid phase materials. It therefore has a strong overlap with
1529:
cf. Chapter 12 of
Elements of X-ray diffraction, B.D. Cullity, Addison-Wesley, 2nd ed. 1977
2522:
2477:
2252:
2072:
2002:
1759:
1739:
1502:
1210:
969:"Layered intercalation compounds: Mechanisms, new methodologies, and advanced applications"
505:
415:
A scanning electron microscope (SEM) used to observe the surface topography and composition
252:
621:
403:
of a material's properties is typically easier for a product with crystalline structures.
193:
8:
2545:
2499:
2424:
2397:
2295:
2277:
2230:
2168:
2064:
2044:
1913:
1908:
1809:
323:
280:
157:
31:
1550:
1506:
1214:
2622:
2588:
2450:
2419:
2300:
2242:
1940:
1923:
1918:
1873:
1836:
1826:
1787:
1630:
1349:
1303:
1241:
1198:
1177:
1047:
998:
837:
728:
425:
399:
176:
27:
1406:
1120:
1078:
652:
639:
2641:
2603:
2568:
2551:
2489:
2407:
2402:
2330:
2315:
2285:
2206:
2173:
2144:
2139:
2114:
2104:
2024:
2012:
1891:
1804:
1656:
1634:
1622:
1614:
1566:
1530:
1471:
1420:
1385:
1353:
1339:
1325:
1307:
1295:
1287:
1246:
1228:
1165:
1155:
1124:
1082:
1051:
1039:
1002:
990:
880:
825:
815:
775:
732:
718:
672:
588:
447:
An X-ray diffractometer (XRD) used to identify the crystalline phases in the material
141:
55:
1371:
363:
information can be obtained during the reaction, which helps identify the products.
2646:
2563:
2218:
2077:
2054:
2007:
1948:
1604:
1558:
1510:
1461:
1453:
1412:
1377:
1331:
1277:
1236:
1218:
1116:
1074:
1029:
980:
942:
929:
Binnewies, Michael; Glaum, Robert; Schmidt, Marcus; Schmidt, Peer (February 2013).
870:
767:
708:
647:
617:
477:
268:
168:
heats the pellet. Tube furnaces are available up to maximum temperatures of 2800C.
985:
968:
2504:
2460:
2455:
2349:
2325:
2159:
2122:
1975:
1965:
1848:
1562:
1515:
1490:
501:
420:
of a certain composition is made by analogy to known material, but this is rare.
348:
279:
is produced by the intercalation method, and this method is the principle behind
232:
153:
152:
of the reactants. If the mixing is not sufficient, we can use techniques such as
39:
1609:
1592:
2388:
2366:
2361:
2356:
2311:
2307:
2290:
2247:
2178:
2039:
2034:
2019:
1831:
1749:
1034:
1017:
509:
360:
123:. Often, the methods prevent defect formation or produce high-purity products.
51:
1416:
1335:
2674:
2593:
2482:
2438:
2163:
1997:
1992:
1985:
1863:
1618:
1475:
1408:
X-Ray
Diffraction Crystallography: Introduction, Examples and Solved Problems
1291:
1266:"Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials"
1232:
1169:
1043:
994:
884:
829:
779:
713:
541:
473:
395:
375:
308:
78:
1282:
1265:
2470:
2320:
2235:
2211:
2201:
2193:
2094:
2029:
1928:
1777:
1652:
1626:
1457:
1299:
1250:
946:
304:
260:
224:
165:
149:
1381:
1149:
1073:, Reactions of Solids with Gases, vol. 21, Elsevier, pp. 1–117,
875:
858:
809:
1868:
771:
489:
312:
300:
271:. The intercalation method was first used in China with the discovery of
188:
103:
59:
1691:
504:
describes the interaction of the nearest neighbouring atoms. Methods of
148:, the reactants are ground together, which decreases size and increases
74:
2494:
1223:
315:, which use a salt with a relatively low melting point as the solvent.
47:
35:
1466:
2556:
1858:
1723:
272:
256:
145:
2578:
545:
544:, they can be characterized by their band structure. It contains a
512:
to probe the electric and magnetic fields around the nucleus. E.g.
411:
352:
332:
276:
91:
2598:
1442:"What Can Electron Microscopy Tell Us Beyond Crystal Structures?"
292:
87:
43:
171:
1675:
1591:
Makuła, Patrycja; Pacia, Michał; Macyk, Wojciech (2018-12-06).
552:
to predict the photochemical properties of the semiconductors.
344:
340:
336:
228:
443:
307:
that is under pressure at temperatures higher than the normal
1549:
Harris, Nadine; Blaber, Martin G.; Schatz, George C. (2016),
1154:. Peter M. Martin (3rd ed.). Amsterdam: Elsevier. 2010.
754:
Mond, Ludwig; Langer, Carl; Quincke, Friedrich (1890-01-01).
164:
are typically synthesized in silica or alumina containers. A
161:
1109:"Chapter 2 Reactions of Solids with Gases other than Oxygen"
928:
374:
is a method widely used for the preparation of coatings and
2573:
1405:
Waseda, Yoshio; Matsubara, Eiichiro; Shinoda, Kozo (2011).
1373:
Energy
Dispersive X-ray Analysis in the Electron Microscope
931:"Chemical Vapor Transport Reactions - A Historical Review"
698:
2583:
1015:
1557:, Dordrecht: Springer Netherlands, pp. 3027–3048,
476:
looks like. An important tool in establishing this are
1404:
1199:"Special Issue: Advances in Chemical Vapor Deposition"
640:"Life and achievements of Carl Wagner, 100th birthday"
1264:
Holder, Cameron F.; Schaak, Raymond E. (2019-07-23).
500:
In contrast to the large structures of crystals, the
1495:
Current
Opinion in Solid State and Materials Science
255:
holding its layers together. The process occurs via
16:
Study of solid materials' properties and composition
966:
331:Many solids react vigorously with gas species like
1548:
1411:. Berlin, Heidelberg: Springer Berlin Heidelberg.
935:Zeitschrift für anorganische und allgemeine Chemie
1491:"X-ray nanobeam diffraction imaging of materials"
1067:"Chapter 1 An Overview of Metal Oxidation Theory"
753:
2672:
1590:
1369:
1064:
175:Tube furnace being used during the synthesis of
1655:and J. Gopalakrishnan. Cambridge U. Press 1997
1440:Zhou, Wuzong; Greer, Heather F. (March 2016).
749:
747:
406:
1707:
760:Journal of the Chemical Society, Transactions
366:
1263:
1115:, vol. 21, Elsevier, pp. 119–149,
1106:
701:"Synthesis Methods in Solid-State Chemistry"
488:and increasingly also, due to the advent of
209:
1551:"Optical Properties of Metal Nanoparticles"
1330:. Cham: Springer International Publishing.
811:Essentials of inorganic materials synthesis
744:
666:
555:
327:Chemical vapour deposition reaction chamber
227:. The Mond process involves heating impure
126:
1714:
1700:
842:: CS1 maint: location missing publisher (
607:
585:Solid State Chemistry and Its Applications
311:. A variation on this theme is the use of
238:
1721:
1608:
1597:The Journal of Physical Chemistry Letters
1514:
1465:
1439:
1370:Bell, Dc; Garratt-Reed, Aj (2003-07-10).
1281:
1240:
1222:
1196:
1033:
984:
874:
712:
651:
814:. Kanishka Biswas. Hoboken, New Jersey.
756:"L.—Action of carbon monoxide on nickel"
442:
410:
322:
192:
182:
170:
73:
1649:New directions in Solid State Chemistry
1488:
1446:European Journal of Inorganic Chemistry
243:
197:Steps involved in molten flux synthesis
2673:
1327:Handbook of Materials Characterization
1323:
856:
637:
578:
576:
1695:
1544:
1542:
1489:Schülli, Tobias U. (September 2018).
1365:
1363:
1319:
1317:
962:
960:
958:
956:
924:
922:
920:
918:
705:Synthesis Methods and Crystallization
694:
692:
690:
688:
622:10.1016/j.progsolidstchem.2007.02.002
527:
259:. Intercalation is further driven by
2629:
1324:Sharma, Surender Kumar, ed. (2018).
803:
801:
799:
797:
795:
793:
791:
789:
667:Cheetham, A. K.; Day, Peter (1988).
633:
631:
624:– via Elsevier Science Direct.
582:
562:Characterisation in material science
438:Energy dispersive X-ray spectroscopy
113:
2653:
1197:Vernardou, Dimitra (January 2020).
807:
573:
463:
381:
286:
13:
1539:
1360:
1314:
953:
915:
685:
495:
458:Selected area electron diffraction
14:
2697:
1668:
1022:npj 2D Materials and Applications
786:
669:Solid State Chemistry: Techniques
638:Martin, Manfred (December 2002).
628:
610:Progress in Solid State Chemistry
131:
2652:
2640:
2628:
2617:
2616:
1674:
857:Pagola, Silvina (January 2023).
550:Ultraviolet-visible spectroscopy
434:transmission electron microscopy
1641:
1584:
1523:
1482:
1433:
1398:
1376:(0 ed.). Garland Science.
1257:
1190:
1142:
1113:Comprehensive Chemical Kinetics
1100:
1071:Comprehensive Chemical Kinetics
1058:
1009:
108:father of solid state chemistry
1555:Encyclopedia of Nanotechnology
891:
850:
660:
601:
540:For non-metallic materials or
318:
1:
1981:Interface and colloid science
1735:Glossary of chemical formulae
1121:10.1016/s0069-8040(08)70007-4
1079:10.1016/s0069-8040(08)70006-2
986:10.1016/j.pmatsci.2019.100631
973:Progress in Materials Science
653:10.1016/S0167-2738(02)00318-1
567:
522:perturbed angular correlation
389:
303:. At times, the solvent is a
81:for use in electronic devices
22:, also sometimes referred as
1563:10.1007/978-94-017-9780-1_22
1553:, in Bhushan, Bharat (ed.),
1516:10.1016/j.cossms.2018.09.003
564:for additional information.
430:scanning electron microscopy
7:
2258:Bioorganometallic chemistry
1745:List of inorganic compounds
1610:10.1021/acs.jpclett.8b02892
407:Compositions and structures
10:
2702:
2184:Dynamic covalent chemistry
2155:Enantioselective synthesis
2135:Physical organic chemistry
2088:Organolanthanide chemistry
1035:10.1038/s41699-021-00211-6
535:surface plasmon resonances
372:Chemical vapour deposition
367:Chemical vapour deposition
186:
121:chemical vapour deposition
69:
2612:
2515:
2276:
2192:
2113:
2063:
1939:
1882:
1773:Electroanalytical methods
1758:
1730:
1417:10.1007/978-3-642-16635-8
1336:10.1007/978-3-319-92955-2
583:West, Anthony R. (2004).
269:electrochemical reactions
215:Chemical vapour transport
210:Chemical vapour transport
64:chemical vapour depostion
2528:Nobel Prize in Chemistry
2444:Supramolecular chemistry
2083:Organometallic chemistry
714:10.5772/intechopen.93337
556:Further characterization
514:electric field gradients
235:to produce pure nickel.
127:High-temperature methods
2466:Combinatorial chemistry
2377:Food physical chemistry
2340:Environmental chemistry
2224:Bioorthogonal chemistry
2150:Retrosynthetic analysis
1971:Chemical thermodynamics
1954:Spectroelectrochemistry
1897:Computational chemistry
1283:10.1021/acsnano.9b05157
587:. John Wiley and Sons.
239:Low-temperature methods
2538:of element discoveries
2384:Agricultural chemistry
2372:Carbohydrate chemistry
2263:Bioinorganic chemistry
2128:Alkane stereochemistry
2073:Coordination chemistry
1902:Mathematical chemistry
1768:Instrumental chemistry
1458:10.1002/ejic.201501342
1182:: CS1 maint: others (
947:10.1002/zaac.201300048
808:Rao, C. N. R. (2015).
518:Mössbauer spectroscopy
448:
416:
328:
291:It is possible to use
198:
179:
100:William Lawrence Bragg
98:in the early 1900s by
82:
26:, is the study of the
2681:Solid-state chemistry
2533:Timeline of chemistry
2430:Post-mortem chemistry
2415:Clandestine chemistry
2345:Atmospheric chemistry
2268:Biophysical chemistry
2100:Solid-state chemistry
2050:Equilibrium chemistry
1959:Photoelectrochemistry
1681:Solid state chemistry
1382:10.4324/9780203483428
876:10.3390/cryst13010124
446:
414:
326:
295:to prepare solids by
281:lithium-ion batteries
196:
183:Molten flux synthesis
174:
96:X-ray crystallography
77:
20:Solid-state chemistry
2523:History of chemistry
2478:Chemical engineering
2253:Bioorganic chemistry
2003:Structural chemistry
1740:List of biomolecules
1683:at Wikimedia Commons
772:10.1039/CT8905700749
506:nuclear spectroscopy
343:. Other solids form
253:intermolecular bonds
244:Intercalation method
2546:The central science
2500:Ceramic engineering
2425:Forensic toxicology
2398:Chemistry education
2296:Radiation chemistry
2278:Interdisciplinarity
2231:Medicinal chemistry
2169:Fullerene chemistry
2045:Microwave chemistry
1914:Molecular mechanics
1909:Molecular modelling
1507:2018COSSM..22..188S
1215:2020Mate...13.4167V
265:acid-base reactions
32:solid-state physics
24:materials chemistry
2589:Chemical substance
2451:Chemical synthesis
2420:Forensic chemistry
2301:Actinide chemistry
2243:Clinical chemistry
1924:Molecular geometry
1919:Molecular dynamics
1874:Elemental analysis
1827:Separation process
1224:10.3390/ma13184167
646:. 152–153: 15–17.
644:Solid State Ionics
528:Optical properties
449:
426:elemental analysis
417:
400:powder diffraction
329:
199:
180:
177:aluminium chloride
83:
2686:Materials science
2668:
2667:
2604:Quantum mechanics
2569:Chemical compound
2552:Chemical reaction
2490:Materials science
2408:General chemistry
2403:Amateur chemistry
2331:Photogeochemistry
2316:Stellar chemistry
2286:Nuclear chemistry
2207:Molecular biology
2174:Polymer chemistry
2145:Organic synthesis
2140:Organic reactions
2105:Ceramic chemistry
2095:Cluster chemistry
2025:Chemical kinetics
2013:Molecular physics
1892:Quantum chemistry
1805:Mass spectrometry
1679:Media related to
1647:cf. Chapter 2 of
1603:(23): 6814–6817.
1572:978-94-017-9779-5
1426:978-3-642-16634-1
1391:978-1-135-33140-5
1345:978-3-319-92954-5
1161:978-0-08-095194-2
821:978-1-118-89267-1
724:978-1-83880-223-3
453:X-ray diffraction
142:mortar and pestle
114:Synthetic methods
56:materials science
2693:
2656:
2655:
2644:
2632:
2631:
2620:
2619:
2564:Chemical element
2219:Chemical biology
2078:Magnetochemistry
2055:Mechanochemistry
2008:Chemical physics
1949:Electrochemistry
1854:Characterization
1716:
1709:
1702:
1693:
1692:
1678:
1663:
1645:
1639:
1638:
1612:
1588:
1582:
1581:
1580:
1579:
1546:
1537:
1527:
1521:
1520:
1518:
1486:
1480:
1479:
1469:
1437:
1431:
1430:
1402:
1396:
1395:
1367:
1358:
1357:
1321:
1312:
1311:
1285:
1276:(7): 7359–7365.
1261:
1255:
1254:
1244:
1226:
1194:
1188:
1187:
1181:
1173:
1146:
1140:
1139:
1138:
1137:
1104:
1098:
1097:
1096:
1095:
1062:
1056:
1055:
1037:
1013:
1007:
1006:
988:
964:
951:
950:
926:
913:
912:
910:
908:
903:
895:
889:
888:
878:
854:
848:
847:
841:
833:
805:
784:
783:
751:
742:
741:
740:
739:
716:
696:
683:
682:
664:
658:
657:
655:
635:
626:
625:
605:
599:
598:
580:
480:techniques like
478:thermal analysis
464:More information
451:Similar to EDX,
428:methods such as
382:Characterization
359:. In that case,
287:Solution methods
154:co-precipitation
2701:
2700:
2696:
2695:
2694:
2692:
2691:
2690:
2671:
2670:
2669:
2664:
2608:
2511:
2505:Polymer science
2461:Click chemistry
2456:Green chemistry
2350:Ocean chemistry
2326:Biogeochemistry
2272:
2188:
2160:Total synthesis
2123:Stereochemistry
2109:
2059:
1976:Surface science
1966:Thermochemistry
1935:
1878:
1849:Crystallography
1754:
1726:
1720:
1671:
1666:
1646:
1642:
1589:
1585:
1577:
1575:
1573:
1547:
1540:
1528:
1524:
1487:
1483:
1438:
1434:
1427:
1403:
1399:
1392:
1368:
1361:
1346:
1322:
1315:
1262:
1258:
1195:
1191:
1175:
1174:
1162:
1148:
1147:
1143:
1135:
1133:
1131:
1105:
1101:
1093:
1091:
1089:
1063:
1059:
1014:
1010:
965:
954:
927:
916:
906:
904:
901:
899:"Tube Furnaces"
897:
896:
892:
855:
851:
835:
834:
822:
806:
787:
752:
745:
737:
735:
725:
697:
686:
679:
665:
661:
636:
629:
606:
602:
595:
581:
574:
570:
558:
530:
502:local structure
498:
496:Local structure
466:
409:
392:
384:
369:
321:
289:
246:
241:
233:carbon monoxide
231:in a stream of
221:
212:
191:
185:
134:
129:
116:
72:
40:crystallography
17:
12:
11:
5:
2699:
2689:
2688:
2683:
2666:
2665:
2663:
2662:
2650:
2638:
2626:
2613:
2610:
2609:
2607:
2606:
2601:
2596:
2591:
2586:
2581:
2576:
2571:
2566:
2561:
2560:
2559:
2549:
2542:
2541:
2540:
2530:
2525:
2519:
2517:
2513:
2512:
2510:
2509:
2508:
2507:
2502:
2497:
2487:
2486:
2485:
2475:
2474:
2473:
2468:
2463:
2458:
2448:
2447:
2446:
2435:
2434:
2433:
2432:
2427:
2417:
2412:
2411:
2410:
2405:
2394:
2393:
2392:
2391:
2389:Soil chemistry
2381:
2380:
2379:
2374:
2367:Food chemistry
2364:
2362:Carbochemistry
2359:
2357:Clay chemistry
2354:
2353:
2352:
2347:
2336:
2335:
2334:
2333:
2328:
2318:
2312:Astrochemistry
2308:Cosmochemistry
2305:
2304:
2303:
2298:
2293:
2291:Radiochemistry
2282:
2280:
2274:
2273:
2271:
2270:
2265:
2260:
2255:
2250:
2248:Neurochemistry
2245:
2240:
2239:
2238:
2228:
2227:
2226:
2216:
2215:
2214:
2209:
2198:
2196:
2190:
2189:
2187:
2186:
2181:
2179:Petrochemistry
2176:
2171:
2166:
2157:
2152:
2147:
2142:
2137:
2132:
2131:
2130:
2119:
2117:
2111:
2110:
2108:
2107:
2102:
2097:
2092:
2091:
2090:
2080:
2075:
2069:
2067:
2061:
2060:
2058:
2057:
2052:
2047:
2042:
2040:Spin chemistry
2037:
2035:Photochemistry
2032:
2027:
2022:
2020:Femtochemistry
2017:
2016:
2015:
2005:
2000:
1995:
1990:
1989:
1988:
1978:
1973:
1968:
1963:
1962:
1961:
1956:
1945:
1943:
1937:
1936:
1934:
1933:
1932:
1931:
1921:
1916:
1911:
1906:
1905:
1904:
1894:
1888:
1886:
1880:
1879:
1877:
1876:
1871:
1866:
1861:
1856:
1851:
1846:
1845:
1844:
1839:
1832:Chromatography
1829:
1824:
1823:
1822:
1817:
1812:
1802:
1801:
1800:
1795:
1790:
1785:
1775:
1770:
1764:
1762:
1756:
1755:
1753:
1752:
1750:Periodic table
1747:
1742:
1737:
1731:
1728:
1727:
1719:
1718:
1711:
1704:
1696:
1690:
1689:
1684:
1670:
1669:External links
1667:
1665:
1664:
1640:
1583:
1571:
1538:
1522:
1501:(5): 188–201.
1481:
1452:(7): 941–950.
1432:
1425:
1397:
1390:
1359:
1344:
1313:
1256:
1189:
1160:
1141:
1129:
1099:
1087:
1057:
1008:
952:
941:(2): 219–229.
914:
890:
849:
820:
785:
743:
723:
707:, IntechOpen,
684:
677:
659:
627:
616:(1–2): 1–133.
600:
593:
571:
569:
566:
557:
554:
542:semiconductors
529:
526:
497:
494:
465:
462:
408:
405:
391:
388:
383:
380:
376:semiconductors
368:
365:
361:stoichiometric
320:
317:
288:
285:
245:
242:
240:
237:
219:
211:
208:
187:Main article:
184:
181:
133:
132:Ceramic method
130:
128:
125:
115:
112:
71:
68:
52:thermodynamics
15:
9:
6:
4:
3:
2:
2698:
2687:
2684:
2682:
2679:
2678:
2676:
2661:
2660:
2651:
2649:
2648:
2643:
2639:
2637:
2636:
2627:
2625:
2624:
2615:
2614:
2611:
2605:
2602:
2600:
2597:
2595:
2594:Chemical bond
2592:
2590:
2587:
2585:
2582:
2580:
2577:
2575:
2572:
2570:
2567:
2565:
2562:
2558:
2555:
2554:
2553:
2550:
2547:
2543:
2539:
2536:
2535:
2534:
2531:
2529:
2526:
2524:
2521:
2520:
2518:
2514:
2506:
2503:
2501:
2498:
2496:
2493:
2492:
2491:
2488:
2484:
2483:Stoichiometry
2481:
2480:
2479:
2476:
2472:
2469:
2467:
2464:
2462:
2459:
2457:
2454:
2453:
2452:
2449:
2445:
2442:
2441:
2440:
2439:Nanochemistry
2437:
2436:
2431:
2428:
2426:
2423:
2422:
2421:
2418:
2416:
2413:
2409:
2406:
2404:
2401:
2400:
2399:
2396:
2395:
2390:
2387:
2386:
2385:
2382:
2378:
2375:
2373:
2370:
2369:
2368:
2365:
2363:
2360:
2358:
2355:
2351:
2348:
2346:
2343:
2342:
2341:
2338:
2337:
2332:
2329:
2327:
2324:
2323:
2322:
2319:
2317:
2313:
2309:
2306:
2302:
2299:
2297:
2294:
2292:
2289:
2288:
2287:
2284:
2283:
2281:
2279:
2275:
2269:
2266:
2264:
2261:
2259:
2256:
2254:
2251:
2249:
2246:
2244:
2241:
2237:
2234:
2233:
2232:
2229:
2225:
2222:
2221:
2220:
2217:
2213:
2210:
2208:
2205:
2204:
2203:
2200:
2199:
2197:
2195:
2191:
2185:
2182:
2180:
2177:
2175:
2172:
2170:
2167:
2165:
2164:Semisynthesis
2161:
2158:
2156:
2153:
2151:
2148:
2146:
2143:
2141:
2138:
2136:
2133:
2129:
2126:
2125:
2124:
2121:
2120:
2118:
2116:
2112:
2106:
2103:
2101:
2098:
2096:
2093:
2089:
2086:
2085:
2084:
2081:
2079:
2076:
2074:
2071:
2070:
2068:
2066:
2062:
2056:
2053:
2051:
2048:
2046:
2043:
2041:
2038:
2036:
2033:
2031:
2028:
2026:
2023:
2021:
2018:
2014:
2011:
2010:
2009:
2006:
2004:
2001:
1999:
1998:Sonochemistry
1996:
1994:
1993:Cryochemistry
1991:
1987:
1986:Micromeritics
1984:
1983:
1982:
1979:
1977:
1974:
1972:
1969:
1967:
1964:
1960:
1957:
1955:
1952:
1951:
1950:
1947:
1946:
1944:
1942:
1938:
1930:
1927:
1926:
1925:
1922:
1920:
1917:
1915:
1912:
1910:
1907:
1903:
1900:
1899:
1898:
1895:
1893:
1890:
1889:
1887:
1885:
1881:
1875:
1872:
1870:
1867:
1865:
1864:Wet chemistry
1862:
1860:
1857:
1855:
1852:
1850:
1847:
1843:
1840:
1838:
1835:
1834:
1833:
1830:
1828:
1825:
1821:
1818:
1816:
1813:
1811:
1808:
1807:
1806:
1803:
1799:
1796:
1794:
1791:
1789:
1786:
1784:
1781:
1780:
1779:
1776:
1774:
1771:
1769:
1766:
1765:
1763:
1761:
1757:
1751:
1748:
1746:
1743:
1741:
1738:
1736:
1733:
1732:
1729:
1725:
1717:
1712:
1710:
1705:
1703:
1698:
1697:
1694:
1687:
1685:
1682:
1677:
1673:
1672:
1662:
1661:0-521-49559-8
1658:
1654:
1650:
1644:
1636:
1632:
1628:
1624:
1620:
1616:
1611:
1606:
1602:
1598:
1594:
1587:
1574:
1568:
1564:
1560:
1556:
1552:
1545:
1543:
1536:
1535:0-201-01174-3
1532:
1526:
1517:
1512:
1508:
1504:
1500:
1496:
1492:
1485:
1477:
1473:
1468:
1463:
1459:
1455:
1451:
1447:
1443:
1436:
1428:
1422:
1418:
1414:
1410:
1409:
1401:
1393:
1387:
1383:
1379:
1375:
1374:
1366:
1364:
1355:
1351:
1347:
1341:
1337:
1333:
1329:
1328:
1320:
1318:
1309:
1305:
1301:
1297:
1293:
1289:
1284:
1279:
1275:
1271:
1267:
1260:
1252:
1248:
1243:
1238:
1234:
1230:
1225:
1220:
1216:
1212:
1208:
1204:
1200:
1193:
1185:
1179:
1171:
1167:
1163:
1157:
1153:
1152:
1145:
1132:
1130:9780444422880
1126:
1122:
1118:
1114:
1110:
1103:
1090:
1088:9780444422880
1084:
1080:
1076:
1072:
1068:
1061:
1053:
1049:
1045:
1041:
1036:
1031:
1027:
1023:
1019:
1012:
1004:
1000:
996:
992:
987:
982:
978:
974:
970:
963:
961:
959:
957:
948:
944:
940:
936:
932:
925:
923:
921:
919:
900:
894:
886:
882:
877:
872:
868:
864:
860:
853:
845:
839:
831:
827:
823:
817:
813:
812:
804:
802:
800:
798:
796:
794:
792:
790:
781:
777:
773:
769:
765:
761:
757:
750:
748:
734:
730:
726:
720:
715:
710:
706:
702:
695:
693:
691:
689:
680:
674:
670:
663:
654:
649:
645:
641:
634:
632:
623:
619:
615:
611:
604:
596:
594:981-253-003-7
590:
586:
579:
577:
572:
565:
563:
553:
551:
547:
543:
538:
536:
525:
523:
519:
515:
511:
508:use specific
507:
503:
493:
491:
487:
483:
479:
475:
474:phase diagram
471:
461:
459:
454:
445:
441:
439:
435:
431:
427:
421:
413:
404:
401:
397:
396:Stoichiometry
387:
379:
377:
373:
364:
362:
358:
354:
350:
346:
342:
338:
334:
325:
316:
314:
310:
309:boiling point
306:
302:
298:
297:precipitation
294:
284:
282:
278:
274:
270:
266:
262:
258:
254:
250:
249:Intercalation
236:
234:
230:
226:
216:
207:
203:
195:
190:
178:
173:
169:
167:
163:
159:
155:
151:
147:
143:
138:
124:
122:
111:
109:
105:
101:
97:
93:
89:
80:
79:Silicon wafer
76:
67:
65:
61:
57:
53:
49:
45:
41:
37:
33:
29:
25:
21:
2657:
2645:
2633:
2621:
2471:Biosynthesis
2321:Geochemistry
2236:Pharmacology
2212:Cell biology
2202:Biochemistry
2099:
2030:Spectroscopy
1929:VSEPR theory
1778:Spectroscopy
1722:Branches of
1653:C. N. R. Rao
1648:
1643:
1600:
1596:
1586:
1576:, retrieved
1554:
1525:
1498:
1494:
1484:
1449:
1445:
1435:
1407:
1400:
1372:
1326:
1273:
1269:
1259:
1209:(18): 4167.
1206:
1202:
1192:
1150:
1144:
1134:, retrieved
1112:
1102:
1092:, retrieved
1070:
1060:
1025:
1021:
1011:
976:
972:
938:
934:
905:. Retrieved
893:
866:
862:
852:
810:
763:
759:
736:, retrieved
704:
668:
662:
643:
613:
609:
603:
584:
559:
539:
531:
499:
490:synchrotrons
469:
467:
450:
422:
418:
393:
385:
370:
330:
313:flux methods
305:hydrothermal
290:
261:ion exchange
247:
225:Mond process
213:
204:
200:
166:tube furnace
162:metal oxides
150:surface area
139:
135:
117:
107:
84:
23:
19:
18:
2659:WikiProject
1884:Theoretical
1869:Calorimetry
1028:(1): 1–21.
766:: 749–753.
319:Gas methods
301:evaporation
189:Flux method
104:Carl Wagner
60:electronics
2675:Categories
2495:Metallurgy
2194:Biological
1760:Analytical
1578:2023-04-15
1467:10023/8104
1136:2023-04-03
1094:2023-04-03
979:: 100631.
869:(1): 124.
738:2023-04-16
678:0198552866
568:References
470:revisiting
432:(SEM) and
390:New phases
347:, such as
48:metallurgy
36:mineralogy
2557:Catalysis
2065:Inorganic
1859:Titration
1724:chemistry
1635:105763124
1619:1948-7185
1476:1434-1948
1354:199491129
1308:198194051
1292:1936-0851
1233:1996-1944
1203:Materials
1178:cite book
1170:670438909
1052:232164576
1044:2397-7132
1003:213438764
995:0079-6425
907:March 30,
885:2073-4352
838:cite book
830:908260711
780:0368-1645
733:225173857
273:porcelain
257:diffusion
146:ball mill
28:synthesis
2623:Category
2579:Molecule
2516:See also
1941:Physical
1627:30990726
1300:31336433
1270:ACS Nano
1251:32961715
863:Crystals
546:band gap
353:ethylene
333:chlorine
293:solvents
277:graphene
275:. Also,
140:Using a
92:platinum
44:ceramics
2635:Commons
2599:Alchemy
2115:Organic
1503:Bibcode
1242:7560419
1211:Bibcode
345:adducts
158:sol-gel
88:zeolite
70:History
2647:Portal
1793:UV-Vis
1659:
1633:
1625:
1617:
1569:
1533:
1474:
1423:
1388:
1352:
1342:
1306:
1298:
1290:
1249:
1239:
1231:
1168:
1158:
1127:
1085:
1050:
1042:
1001:
993:
883:
828:
818:
778:
731:
721:
675:
591:
510:nuclei
341:oxygen
339:, and
337:iodine
299:or by
229:nickel
1820:MALDI
1788:Raman
1631:S2CID
1350:S2CID
1304:S2CID
1048:S2CID
999:S2CID
902:(PDF)
729:S2CID
2574:Atom
1842:HPLC
1657:ISBN
1623:PMID
1615:ISSN
1567:ISBN
1531:ISBN
1472:ISSN
1450:2016
1421:ISBN
1386:ISBN
1340:ISBN
1296:PMID
1288:ISSN
1247:PMID
1229:ISSN
1184:link
1166:OCLC
1156:ISBN
1125:ISBN
1083:ISBN
1040:ISSN
991:ISSN
909:2023
881:ISSN
844:link
826:OCLC
816:ISBN
776:ISSN
719:ISBN
673:ISBN
589:ISBN
520:and
156:and
90:and
58:and
2584:Ion
1815:ICP
1798:NMR
1605:doi
1559:doi
1511:doi
1462:hdl
1454:doi
1413:doi
1378:doi
1332:doi
1278:doi
1237:PMC
1219:doi
1117:doi
1075:doi
1030:doi
981:doi
977:109
943:doi
939:639
871:doi
768:doi
709:doi
648:doi
618:doi
486:DTA
484:or
482:DSC
357:TGA
351:or
267:or
144:or
2677::
2314:/
2310:/
2162:/
1837:GC
1810:EI
1783:IR
1651:.
1629:.
1621:.
1613:.
1599:.
1595:.
1565:,
1541:^
1509:.
1499:22
1497:.
1493:.
1470:.
1460:.
1448:.
1444:.
1419:.
1384:.
1362:^
1348:.
1338:.
1316:^
1302:.
1294:.
1286:.
1274:13
1272:.
1268:.
1245:.
1235:.
1227:.
1217:.
1207:13
1205:.
1201:.
1180:}}
1176:{{
1164:.
1123:,
1111:,
1081:,
1069:,
1046:.
1038:.
1024:.
1020:.
997:.
989:.
975:.
971:.
955:^
937:.
933:.
917:^
879:.
867:13
865:.
861:.
840:}}
836:{{
824:.
788:^
774:.
764:57
762:.
758:.
746:^
727:,
717:,
703:,
687:^
671:.
642:.
630:^
614:36
612:.
575:^
524:.
349:CO
335:,
283:.
263:,
218:Cl
110:.
54:,
50:,
46:,
42:,
38:,
34:,
2548:"
2544:"
1715:e
1708:t
1701:v
1637:.
1607::
1601:9
1561::
1519:.
1513::
1505::
1478:.
1464::
1456::
1429:.
1415::
1394:.
1380::
1356:.
1334::
1310:.
1280::
1253:.
1221::
1213::
1186:)
1172:.
1119::
1077::
1054:.
1032::
1026:5
1005:.
983::
949:.
945::
911:.
887:.
873::
846:)
832:.
782:.
770::
711::
681:.
656:.
650::
620::
597:.
220:2
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.