20:
888:
898:
36:
339:. By ignoring the electrons Rutherford also ignores any potential implications for atomic spectroscopy for chemistry. Rutherford himself did not press the case for his atomic model in the following years: his own 1913 book on "Radioactive substances and their radiations" only mentions the atom twice; other books by other authors around this time focus on Thomson's model.
126:, primarily in 1904-06. He produced an elaborate mechanical model of the electrons moving in concentric rings, but the positive charge needed to balance the negative electrons was a simple sphere of uniform charge and unknown composition. Between 1904 and 1910 Thomson developed formulae for the deflection of fast
38:
262:) was 79, and Rutherford had modelled the charge to be about +100 units (he had actually suggested 98 units of positive charge, to make half of 196). Thus, Rutherford did not formally suggest the two numbers (periodic table place, 79, and nuclear charge, 98 or 100) might be exactly the same.
141:
like model for atoms, with very strongly charged "positive suns" surrounded by "corpuscles, a kind of small negative planets", where the word "corpuscles" refers to what we now call electrons. Perrin discussed how this hypothesis might related to important then unexplained phenomena like the
42:
41:
37:
43:
83:
of the atom was incorrect. Rutherford's new model for the atom, based on the experimental results, contained new features of a relatively high central charge concentrated into a very small volume in comparison to the rest of the atom and with this central volume containing most of the
196:
in
Rutherford's lab showed that alpha particles could occasionally be reflected from gold foils. If Thomson was correct, the beam would go through the gold foil with very small deflections. In the experiment most of the beam passed through the foil, but a few were deflected.
249:
u (roughly 1/2 of it, in
Rutherford's model). For gold, this mass number is 197 (not then known to great accuracy) and was therefore modelled by Rutherford to be possibly 196 u. However, Rutherford did not attempt to make the direct connection of central charge to
229:
central charge would need to be less (how much less could not be told) than 3.4 × 10 metres. This was in a gold atom known to be 10 metres or so in radius—a very surprising finding, as it implied a strong central charge less than 1/3000th of the diameter of the atom.
269:
suggested that the nuclear charge and atomic weight were not connected, clearing the way for the idea that atomic number and nuclear charge were the same. This idea was quickly taken up by
Rutherford's team and was confirmed experimentally within two years by
40:
233:
The
Rutherford model served to concentrate a great deal of the atom's charge and mass to a very small core, but did not attribute any structure to the remaining electrons and remaining atomic mass. It did mention the atomic model of
165:
as an analog. The rings consisted of a large number of particles that repelled each other but were attracted to a large central charge. This charge was calculated to be 10,000 times the charge of the ring particles for stability.
200:
In a May 1911 paper, Rutherford presented his own physical model for subatomic structure, as an interpretation for the unexpected experimental results. In it, the atom is made up of a central charge (this is the modern
361:. Scientists eventually discovered that atoms have a positively charged nucleus (with an atomic number of charges) in the center, with a radius of about 1.2 × 10 meters × . Electrons were found to be even smaller.
311:
The mass of heavy atoms such as gold is mostly concentrated in the central charge region, since calculations show it is not deflected or moved by the high speed alpha particles, which have very high
39:
188:, a form of radiation Rutherford discovered in 1899. These experiments demonstrated that alpha particles "scattered" or bounced off atoms in ways unlike Thomson's model predicted. In 1908 and 1910,
225:
Using only energetic considerations of how far particles of known speed would be able to penetrate toward a central charge of 100 e, Rutherford was able to calculate that the radius of his
738:
Andrade, Edward
Neville Da Costa. "The Rutherford Memorial Lecture, 1957." Proceedings of the Royal Society of London. Series A. Mathematical and Physical Sciences 244.1239 (1958): 437-455.
205:, though Rutherford did not use the term "nucleus" in his paper). Rutherford only committed himself to a small central region of very high positive or negative charge in the atom.
114:'s model was the first of these models to be based on experimentally detected subatomic particles. In the same paper that Thomson announced his results on "corpuscle" nature of
346:
arrived as a post-doctoral student in
Manchester at Rutherford's invitation. Bohr dropped his work on the Thomson model in favor of Rutherford's nuclear model, developing the
756:
130:
from his atomic model for comparison to experiment. Similar work by
Rutherford using alpha particles would eventually show Thomson's model could not be correct.
318:
The atom itself is about 100,000 (10) times the diameter of the nucleus. This could be related to putting a grain of sand in the middle of a
238:, in which the electrons are arranged in one or more rings, with the specific metaphorical structure of the stable rings of Saturn. The
380:
Models and modelers of hydrogen: Thales, Thomson, Rutherford, Bohr, Sommerfeld, Goudsmit, Heisenberg, Schrödinger, Dirac, Sallhofer
783:
170:
showed in 1904 that
Nagaoka's model could not be consistent with results of atomic spectroscopy and the model fell out of favor.
941:
936:
723:
624:
505:
387:
292:
Much of an atom's positive charge is concentrated in a relatively tiny volume at the center of the atom, known today as the
751:
296:. The magnitude of this charge is proportional to (up to a charge number that can be approximately half of) the atom's
331:
Rutherford's new atom model caused no reaction at first. Rutherford explicitly ignores the electrons, only mentioning
520:
179:
209:
For concreteness, consider the passage of a high speed α particle through an atom having a positive central charge
415:. RePoSS: Research Publications on Science Studies 10. Aarhus: Centre for Science Studies, University of Aarhus.
245:
The
Rutherford paper suggested that the central charge of an atom might be "proportional" to its atomic mass in
828:
358:
357:
After
Rutherford's discovery, subsequent research determined the atomic structure which led to Rutherford's
72:
776:
931:
293:
122:, he began speculating on atomic models composed of electrons. He developed his model, now called the
814:
347:
926:
792:
105:
575:
354:
into the model of the atom, allowing prediction of electronic spectra and concepts of chemistry.
769:
614:
266:
167:
533:
486:
Giliberti, Marco; Lovisetti, Luisa (2024). "Rutherford's Hypothesis on the Atomic Structure".
319:
286:
133:
Also among the early models where "planetary" or Solar System-like models. In a 1901 paper,
649:
143:
134:
8:
304:. This concentrated central mass and charge is responsible for deflecting both alpha and
653:
534:"'Rutherford's experiment' on alpha particles scattering: the experiment that never was"
487:
872:
835:
807:
459:
239:
123:
80:
676:
897:
719:
696:
620:
595:
553:
501:
451:
383:
351:
162:
64:
576:"LXXIX. The scattering of α and β particles by matter and the structure of the atom"
412:
110:
Throughout the 1800's speculative ideas about atoms where discussed and published.
19:
905:
688:
657:
587:
545:
493:
443:
246:
147:
692:
336:
332:
235:
158:
497:
184:
Rutherford's nuclear model of the atom grew out of a series of experiments with
23:
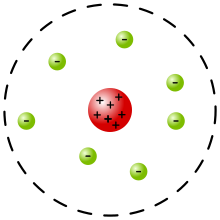
Schematic diagram Rutherford's atom: electrons in green and nucleus in red. The
901:
891:
866:
580:
The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science
549:
489:
Old Quantum Theory and Early Quantum Mechanics. Challenges in Physics Education
282:
259:
202:
193:
185:
89:
48:
24:
591:
476:
Perrin J (1901) Les hypothèses moléculaires. Revue Scientifique 15(15):449–461
920:
700:
599:
557:
455:
305:
271:
251:
154:. Perrin later credited Rutherford with the discovery of the nuclear model.
151:
76:
821:
315:
in comparison to electrons, but not with regard to a heavy atom as a whole.
138:
115:
350:
over the next several years. Eventually Bohr incorporated early ideas of
297:
189:
85:
463:
431:
848:
447:
343:
127:
111:
93:
661:
52:
51:
shown expanded more than 10,000 times its size relative to the atom;
28:
27:
shown expanded more than 10,000 times its size relative to the atom;
312:
301:
119:
761:
75:
in 1909, which suggested, upon Rutherford's 1911 analysis, that
47:
3D animation of an atom incorporating the Rutherford model. The
640:
Constan, Zach (2010). "Learning Nuclear Science with Marbles".
677:"Early atomic models – from mechanical to quantum (1904–1913)"
300:—the remaining mass is now known to be mostly attributed to
432:"The Scattering of α and β Particles and Rutherford's Atom"
226:
68:
92:. The Rutherford model was subsequently superseded by the
242:
of J. J. Thomson also had rings of orbiting electrons.
16:
Atomic model devised to explain alpha particle scattering
716:
Inward bound: of matter and forces in the physical world
492:. Cham: Springer Nature Switzerland. pp. 229–268.
382:. Singapore ; River Edge, NJ: World Scientific.
525:
342:
The impact of Rutherford's nuclear model came after
413:
Before Bohr: Theories of atomic structure 1850-1913
173:
485:
612:
531:
479:
326:
918:
569:
567:
707:
514:
425:
423:
421:
777:
718:(Reprint ed.). Oxford: Clarendon Press
564:
217:, and surrounded by a compensating charge of
616:College Physics: Reasoning and Relationships
371:
732:
633:
606:
418:
407:
405:
403:
401:
399:
118:, an event considered the discovery of the
784:
770:
573:
668:
470:
377:
137:used Thomson's discovery in a proposed a
429:
396:
34:
18:
757:Rutherford's Model by Kyushu University
639:
532:Leone, M; Robotti, N; Verna, G (2018).
521:1926 Lecture for Nobel Prize in Physics
919:
765:
752:Rutherford's Model by Raymond College
674:
436:Archive for History of Exact Sciences
157:A somewhat similar model proposed by
713:
619:. Cengage Learning. pp. 1051–.
613:Nicholas Giordano (1 January 2012).
258:time merely its place number in the
88:; this region would be known as the
285:does not (substantially) influence
254:, since gold's "atomic number" (at
13:
14:
953:
791:
745:
180:Rutherford scattering experiments
896:
887:
886:
174:Experimental basis for the model
681:The European Physical Journal H
327:Contribution to modern science
277:These are the key indicators:
1:
364:
99:
942:Obsolete theories in physics
937:Foundational quantum physics
875:(relativistic quantum model)
55:have no measurable diameter.
31:have no measurable diameter.
7:
574:Rutherford, E. (May 1911).
498:10.1007/978-3-031-57934-9_6
378:Lakhtakia, A., ed. (1996).
10:
958:
693:10.1140/epjh/e2012-30009-7
675:Baily, C. (January 2013).
430:Heilbron, John L. (1968).
177:
103:
71:. Rutherford directed the
882:
858:
815:vortex theory of the atom
799:
592:10.1080/14786440508637080
411:Helge Kragh (Oct. 2010).
287:alpha particle scattering
73:Geiger–Marsden experiment
550:10.1088/1361-6552/aaa353
106:History of atomic theory
714:Pais, Abraham (2002).
267:Antonius van den Broek
223:
56:
32:
810:(billiard ball model)
348:Rutherford–Bohr model
207:
46:
22:
867:electron cloud model
824:(cubical atom model)
359:gold foil experiment
144:photoelectric effect
135:Jean Baptiste Perrin
851:(old quantum model)
654:2010PhTea..48..114C
642:The Physics Teacher
247:hydrogen mass units
873:Dirac–Gordon model
836:plum pudding model
448:10.1007/BF00411591
240:plum pudding model
124:plum pudding model
81:plum pudding model
57:
33:
932:Ernest Rutherford
914:
913:
844:(planetary model)
831:(Saturnian model)
725:978-0-19-851997-3
662:10.1119/1.3293660
626:978-1-285-22534-0
538:Physics Education
507:978-3-031-57933-2
389:978-981-02-2302-1
352:quantum mechanics
65:Ernest Rutherford
44:
949:
900:
890:
889:
842:Rutherford model
786:
779:
772:
763:
762:
739:
736:
730:
729:
711:
705:
704:
672:
666:
665:
637:
631:
630:
610:
604:
603:
586:(125): 669–688.
571:
562:
561:
529:
523:
518:
512:
511:
483:
477:
474:
468:
467:
427:
416:
409:
394:
393:
375:
168:George A. Schott
148:emission spectra
61:Rutherford model
45:
957:
956:
952:
951:
950:
948:
947:
946:
927:1911 in science
917:
916:
915:
910:
878:
854:
800:Historic models
795:
790:
748:
743:
742:
737:
733:
726:
712:
708:
673:
669:
638:
634:
627:
611:
607:
572:
565:
530:
526:
519:
515:
508:
484:
480:
475:
471:
428:
419:
410:
397:
390:
376:
372:
367:
337:Saturnian model
333:Hantaro Nagaoka
329:
236:Hantaro Nagaoka
186:alpha particles
182:
176:
159:Hantaro Nagaoka
108:
102:
67:to describe an
63:was devised by
35:
17:
12:
11:
5:
955:
945:
944:
939:
934:
929:
912:
911:
909:
908:
902:Portal:Physics
894:
892:Category:Atoms
883:
880:
879:
877:
876:
869:
862:
860:
859:Current models
856:
855:
853:
852:
845:
838:
832:
825:
818:
811:
803:
801:
797:
796:
789:
788:
781:
774:
766:
760:
759:
754:
747:
746:External links
744:
741:
740:
731:
724:
706:
667:
648:(2): 114–117.
632:
625:
605:
563:
524:
513:
506:
478:
469:
442:(4): 247–307.
417:
395:
388:
369:
368:
366:
363:
328:
325:
324:
323:
320:football field
316:
309:
290:
283:electron cloud
260:periodic table
203:atomic nucleus
194:Ernest Marsden
178:Main article:
175:
172:
163:Saturn's rings
128:beta particles
104:Main article:
101:
98:
90:atomic nucleus
49:atomic nucleus
25:atomic nucleus
15:
9:
6:
4:
3:
2:
954:
943:
940:
938:
935:
933:
930:
928:
925:
924:
922:
907:
903:
899:
895:
893:
885:
884:
881:
874:
870:
868:
864:
863:
861:
857:
850:
846:
843:
839:
837:
833:
830:
829:Nagaoka model
826:
823:
819:
816:
812:
809:
805:
804:
802:
798:
794:
793:Atomic models
787:
782:
780:
775:
773:
768:
767:
764:
758:
755:
753:
750:
749:
735:
727:
721:
717:
710:
702:
698:
694:
690:
686:
682:
678:
671:
663:
659:
655:
651:
647:
643:
636:
628:
622:
618:
617:
609:
601:
597:
593:
589:
585:
581:
577:
570:
568:
559:
555:
551:
547:
544:(3): 035003.
543:
539:
535:
528:
522:
517:
509:
503:
499:
495:
491:
490:
482:
473:
465:
461:
457:
453:
449:
445:
441:
437:
433:
426:
424:
422:
414:
408:
406:
404:
402:
400:
391:
385:
381:
374:
370:
362:
360:
355:
353:
349:
345:
340:
338:
334:
321:
317:
314:
310:
307:
303:
299:
295:
291:
288:
284:
280:
279:
278:
275:
273:
272:Henry Moseley
268:
263:
261:
257:
253:
252:atomic number
248:
243:
241:
237:
231:
228:
222:
220:
216:
212:
206:
204:
198:
195:
191:
187:
181:
171:
169:
164:
161:in 1904 used
160:
155:
153:
152:radioactivity
149:
145:
140:
136:
131:
129:
125:
121:
117:
113:
107:
97:
95:
91:
87:
82:
78:
77:J. J. Thomson
74:
70:
66:
62:
54:
50:
30:
26:
21:
841:
817:(knot model)
808:Dalton model
734:
715:
709:
684:
680:
670:
645:
641:
635:
615:
608:
583:
579:
541:
537:
527:
516:
488:
481:
472:
439:
435:
379:
373:
356:
341:
330:
276:
264:
255:
244:
232:
224:
218:
214:
210:
208:
199:
183:
156:
139:Solar System
132:
116:cathode rays
109:
60:
58:
822:Lewis model
687:(1): 1–38.
298:atomic mass
281:The atom's
221:electrons.
190:Hans Geiger
86:atom's mass
921:Categories
849:Bohr model
365:References
344:Niels Bohr
308:particles.
112:JJ Thomson
100:Background
94:Bohr model
906:Chemistry
701:2102-6459
600:1941-5982
558:0031-9120
456:0003-9519
53:electrons
29:electrons
464:41133273
313:momentum
302:neutrons
265:In 1913
120:electron
650:Bibcode
294:nucleus
722:
699:
623:
598:
556:
504:
462:
454:
386:
213:
150:, and
871:1928
865:1926
847:1913
840:1911
834:1904
827:1904
820:1902
813:1867
806:1804
460:JSTOR
720:ISBN
697:ISSN
621:ISBN
596:ISSN
554:ISSN
502:ISBN
452:ISSN
384:ISBN
306:beta
256:that
227:gold
192:and
69:atom
59:The
689:doi
658:doi
588:doi
546:doi
494:doi
444:doi
335:'s
146:,
79:'s
923::
904:/
695:.
685:38
683:.
679:.
656:.
646:48
644:.
594:.
584:21
582:.
578:.
566:^
552:.
542:53
540:.
536:.
500:.
458:.
450:.
438:.
434:.
420:^
398:^
274:.
96:.
785:e
778:t
771:v
728:.
703:.
691::
664:.
660::
652::
629:.
602:.
590::
560:.
548::
510:.
496::
466:.
446::
440:4
392:.
322:.
289:.
219:N
215:e
211:N
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.