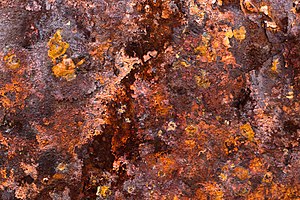157:
1412:
532:
1393:
1377:
896:
1080:
994:
43:
512:
933:
140:
1286:
2045:
1711:
1099:, or wax tapes that isolate the iron from the environment. Large structures with enclosed box sections, such as ships and modern automobiles, often have a wax-based product (technically a "slushing oil") injected into these sections. Such treatments usually also contain rust inhibitors. Covering steel with concrete can provide some protection to steel because of the
979:" alloys such as Cor-Ten rust at a much slower rate than normal, because the rust adheres to the surface of the metal in a protective layer. Designs using this material must include measures that avoid worst-case exposures since the material still continues to rust slowly even under near-ideal conditions.
1301:
Rust is associated with the degradation of iron-based tools and structures. As rust has a much higher volume than the originating mass of iron, its buildup can also cause failure by forcing apart adjacent parts — a phenomenon sometimes known as "rust packing". It was the cause of the collapse of the
603:
species are formed. Unlike ferrous oxides, the hydroxides do not adhere to the bulk metal. As they form and flake off from the surface, fresh iron is exposed, and the corrosion process continues until either all of the iron is consumed or all of the oxygen, water, carbon dioxide or sulfur dioxide in
1125:
over the iron joints for protection from seismic shocks as well as from corrosion. This method was successful for the 2500-year-old structure, but in less than a century the crude repairs were in imminent danger of collapse. When only temporary protection is needed for storage or transport, a thin
559:
and cracks in any exposed metal. The hydrogen atoms present in water molecules can combine with other elements to form acids, which will eventually cause more metal to be exposed. If chloride ions are present, as is the case with saltwater, the corrosion is likely to occur more quickly. Meanwhile,
1057:
Cathodic protection is a technique used to inhibit corrosion on buried or immersed structures by supplying an electrical charge that suppresses the electrochemical reaction. If correctly applied, corrosion can be stopped completely. In its simplest form, it is achieved by attaching a sacrificial
543:
Rust is a general name for a complex of oxides and hydroxides of iron, which occur when iron or some alloys that contain iron are exposed to oxygen and moisture for a long period of time. Over time, the oxygen combines with the metal, forming new compounds collectively called rust, in a process
906:
Because of the widespread use and importance of iron and steel products, the prevention or slowing of rust is the basis of major economic activities in a number of specialized technologies. A brief overview of methods is presented here; for detailed coverage, see the cross-referenced articles.
1597:
1137:
Special anti-seize lubricant mixtures are available and are applied to metallic threads and other precision machined surfaces to protect them from rust. These compounds usually contain grease mixed with copper, zinc, or aluminium powder, and other proprietary ingredients.
1357:
1121:, in 1898, but caused extensive damage to the marble by the rusting and swelling of unprotected iron. The ancient Greek builders had used a similar fastening system for the marble blocks during construction, however, they also poured molten
1234:. Overvoltage also produces small amounts of ozone, which is highly toxic, so a low voltage phone charger is a far safer source of DC current. The effects of hydrogen on global warming have also recently come under scrutiny.
1167:
Corrosion inhibitors, such as gas-phase or volatile inhibitors, can be used to prevent corrosion inside sealed systems. They are not effective when air circulation disperses them, and brings in fresh oxygen and moisture.
580:, the iron tends to rust more quickly, as a result of chemical reactions. Iron metal is relatively unaffected by pure water or by dry oxygen. As with other metals, like aluminium, a tightly adhering oxide coating, a
1028:; aluminium will migrate to cover scratches and thus provide protection for a longer period. These approaches rely on the aluminium and zinc oxides protecting a once-scratched surface, rather than oxidizing as a
1152:
Bluing is a technique that can provide limited resistance to rusting for small steel items, such as firearms; for it to be successful, a water-displacing oil is rubbed onto the blued steel and other steel .
819:
From the above equations, it is also seen that the corrosion products are dictated by the availability of water and oxygen. With limited dissolved oxygen, iron(II)-containing materials are favoured, including
471:, causes the object to have a thin coating of rust over the top. If kept in low relative humidity, it makes the "stable" layer protective to the iron below, but not to the extent of other oxides such as
560:
the oxygen atoms combine with metallic atoms to form the destructive oxide compound. These iron compounds are brittle and crumbly and replace strong metallic iron, reducing the strength of the object.
1411:
1021:
plating is preferred instead of the underlying protected metal. The protective zinc layer is consumed by this action, and thus galvanization provides protection only for a limited period of time.
625:
from iron to oxygen. The iron is the reducing agent (gives up electrons) while the oxygen is the oxidizing agent (gains electrons). The rate of corrosion is affected by water and accelerated by
1858:
2231:. C. L. Page, P. B. Bamforth, J. W. Figg, International Symposium on Corrosion of Reinforcement in Concrete Construction. Cambridge: Royal Society of Chemistry, Information Services. 1996.
1062:
than the iron or steel, commonly zinc, aluminium, or magnesium. The sacrificial anode will eventually corrode away, ceasing its protective action unless it is replaced in a timely manner.
1789:
The steel is allowed to rust and because of its alloy composition rusts more slowly than conventional steel and the rust forms a protective coating slowing the rate of future corrosion
156:
1817:
For atmospheric corrosion, refer to the
Galvanizers Association Millennium Map of average zinc corrosion rates. About 50% of England and Wales has a rate of under 1 μm/year
1695:
914:
to air and water, therefore the interior metallic iron beneath a rust layer continues to corrode. Rust prevention thus requires coatings that preclude rust formation.
2156:
2029:
1392:
902:
is a group of steel alloys which were developed to eliminate the need for painting, and form a stable rust-like appearance after several years' exposure to weather.
1106:
environment at the steel–concrete interface. However, rusting of steel in concrete can still be a problem, as expanding rust can fracture concrete from within.
607:
When iron rusts, the oxides take up more volume than the original metal; this expansion can generate enormous forces, damaging structures made with iron. See
491:
from 2.5 to 2.2 billion years ago. Afterwards, rust soon uplifted iron metals toward the ocean surface. They would subsequently transform into foundations of
1734:
1226:
as a power source in which the positive terminal is clamped to the anode and the negative terminal is clamped to the object to be treated which becomes the
1039:
Typical galvanization of steel products that are to be subjected to normal day-to-day weathering in an outside environment consists of a hot-dipped 85
1443:
for slow decay due to neglect, since it gradually converts robust iron and steel metal into a soft crumbling powder. A wide section of the industrialized
1065:
Cathodic protection can also be provided by using an applied electrical current. This would then be known as ICCP Impressed
Current Cathodic Protection.
409:
Given sufficient time, any iron mass, in the presence of water and oxygen, could eventually convert entirely to rust. Surface rust is commonly flaky and
1230:. Hydrogen and oxygen gases are produced at the cathode and anode respectively. This mixture is flammable/explosive. Care should also be taken to avoid
1555:
1356:
1865:
1333:
in 2003, largely because the central base bolts holding the structure to the ground had rusted away, leaving the bridge anchored by gravity alone.
1043:
zinc coating. Under normal weather conditions, this will deteriorate at a rate of 1 μm per year, giving approximately 85 years of protection.
887:. The solution detects both Fe ions and hydroxyl ions. Formation of Fe ions and hydroxyl ions are indicated by blue and pink patches respectively.
2002:
1339:
is also vulnerable to rust damage. Internal pressure caused by expanding corrosion of concrete-covered steel and iron can cause the concrete to
1058:
anode, thereby making the iron or steel the cathode in the cell formed. The sacrificial anode must be made from something with a more negative
1837:
1529:
555:
for the rusting process is water. Iron or steel structures might appear to be solid, but water molecules can penetrate the microscopic
548:
reaction specifically occurring with iron. Other metals also corrode via similar oxidation, but such corrosion is not called rusting.
675:, this process is strongly affected by the presence of acid. Likewise, the corrosion of most metals by oxygen is accelerated at low
1032:
as in traditional galvanized coatings. In some cases, such as very aggressive environments or long design life, both zinc and a
1924:
2210:
2193:
1900:
1581:
2152:
2067:
1977:
1202:
can be done in a home workshop using simple materials such as a plastic bucket filled with an electrolyte consisting of
487:
It was assumed that rust, made by dissolved oxygen with iron in the oceans, began to sink beneath the seafloor, forming
1376:
2278:
2236:
2039:
1705:
1645:
1591:
126:
2202:
Corrosion engineering handbook. Fundamentals of metallic corrosion : atmospheric and media corrosion of metals
107:
1726:
231:
1459:, and other manufacturers, has experienced harsh economic cutbacks that have caused the region to be dubbed the "
467:, is an example. Although rusting is generally a negative aspect of iron, a particular form of rusting, known as
79:
1678:
1017:
to the steel surface in case of damage of the zinc layer. In more corrosive environments (such as salt water),
448:, and form under different circumstances. Other forms of rust include the result of reactions between iron and
64:
1544:
679:. Providing the electrons for the above reaction is the oxidation of iron that may be described as follows:
86:
2115:
1801:
1614:
1343:, creating severe structural problems. It is one of the most common failure modes of reinforced concrete
884:
1466:
In music, literature, and art, rust is associated with images of faded glory, neglect, decay, and ruin.
531:
2260:
93:
1306:
in 1983, when the bearings rusted internally and pushed one corner of the road slab off its support.
391:
1186:
Rust can be avoided by controlling the moisture in the atmosphere. An example of this is the use of
1756:"Formation of stable passive film on stainless steel by electrochemical deposition of polypyrrole"
1321:
collapsed in less than a minute, killing 46 drivers and passengers on the bridge at the time. The
236:
1403:
941:
581:
414:
75:
53:
17:
1829:
1521:
1231:
710:
1001:
Galvanization consists of an application on the object to be protected of a layer of metallic
865:. The nature of rust changes with time, reflecting the slow rates of the reactions of solids.
2116:"Global warming potential (GWP) for hydrogen: Sensitivities, uncertainties and meta-analysis"
1694:
Ramaswamy, Hosahalli S.; Marcotte, Michele; Sastry, Sudhir; Abdelrahim, Khalid (2014-02-14).
1475:
500:
488:
375:
60:
31:
1218:, another laid across the top of the bucket to act as a support for suspending the object,
753:
8:
1456:
1426:
1336:
1303:
1162:
1059:
1052:
1014:
1006:
945:
868:
Furthermore, these complex processes are affected by the presence of other ions, such as
588:
layer to rust results from the combined action of two agents, usually oxygen and water.
2296:
2254:
1448:
746:
729:
516:
173:
1013:. Zinc is traditionally used because it is cheap, adheres well to steel, and provides
2274:
2242:
2232:
2216:
2206:
2189:
2035:
1906:
1896:
1755:
1701:
1674:
1641:
1587:
1543:
Ankersmit, Bart; Griesser-Stermscheg, Martina; Selwyn, Lindsie; Sutherland, Susanne.
1399:
1318:
1258:
1029:
556:
1950:
1773:
997:
Interior rust in old galvanized iron water pipes can result in brown and black water
441:
undergo similar corrosion, but the resulting oxides are not commonly called "rust".
2181:
2131:
2127:
1666:
1444:
1203:
976:
927:
899:
872:, which serve as electrolytes which accelerate rust formation, or combine with the
621:
The rusting of iron is an electrochemical process that begins with the transfer of
584:, protects the bulk iron from further oxidation. The conversion of the passivating
287:
282:
100:
1246:
1223:
923:
539:
temperature of 1200°C. Rapid oxidation occurs when heated steel is exposed to air
472:
277:
1890:
1238:
1147:
1118:
1010:
821:
694:
600:
596:
592:
328:
272:
252:
202:
164:
2090:
1130:
can be applied to an iron surface. Such treatments are extensively used when "
697:
also occurs in the presence of water and is crucial to the formation of rust:
633:
on the corrosion of automobiles. The key reaction is the reduction of oxygen:
2301:
2290:
2220:
1910:
1383:
1363:
1322:
1314:
1310:
1294:
1131:
988:
895:
585:
520:
2246:
1670:
1079:
993:
883:
The onset of rusting can also be detected in the laboratory with the use of
1661:
Gräfen, H.; Horn, E. M.; Schlecker, H.; Schindler, H. (2000). "Corrosion".
1326:
1199:
1177:
1074:
445:
403:
371:
333:
323:
262:
257:
2200:
2059:
1951:"Rust Wedge: Expanding rust is a force strong enough to shatter concrete"
1452:
1250:
1242:
1219:
969:
626:
267:
211:
1542:
1495:
1222:
to suspend the object in the solution from the horizontal rebar, and a
1187:
961:
911:
577:
524:
464:
430:
347:
318:
292:
226:
188:
1134:" a steel ship, automobile, or other equipment for long-term storage.
1460:
1280:
1262:
1207:
1181:
1127:
1114:
965:
949:
873:
829:
825:
669:
662:
630:
552:
476:
426:
399:
363:
313:
308:
183:
178:
1545:"Rust Never Sleeps: Recognizing Metals and Their Corrosion Products"
42:
2268:
1440:
1266:
1100:
953:
622:
573:
457:
449:
216:
193:
535:
Rust scale forming and flaking off from a steel bar heated to its
511:
1693:
1330:
1254:
1227:
1096:
1092:
1033:
1018:
869:
536:
410:
221:
30:
This article is about the chemical compound. For other uses, see
1265:
agents as in some commercial formulations or even a solution of
1344:
1110:
932:
841:
527:, causing surface breakdown, cracking, and flaking of the metal
460:
444:
Several forms of rust are distinguishable both visually and by
418:
359:
139:
2091:"Exploding Hydrogen Balloons with Different Amounts of Oxygen"
1083:
Flaking paint, exposing a patch of surface rust on sheet metal
1422:
1418:
1340:
1285:
1215:
1211:
1088:
877:
808:
788:
769:
650:
545:
496:
453:
438:
434:
367:
351:
1802:"Steel corrosion protection - Durability - Structural steel"
1660:
1109:
As a closely related example, iron clamps were used to join
1040:
568:
When iron is in contact with water and oxygen, it rusts. If
1367:
1122:
1002:
957:
880:
of iron to precipitate a variety of Ca, Fe, O, OH species.
779:
760:
569:
492:
417:
protection to the underlying iron, unlike the formation of
355:
1249:
which combines with rust; removed with organic acids like
1190:
packets to control humidity in equipment shipped by sea.
672:
1087:
Rust formation can be controlled with coatings, such as
1103:
676:
1237:
Rust may be treated with commercial products known as
1036:
are applied to provide enhanced corrosion protection.
1024:
More modern coatings add aluminium to the coating as
2229:
Corrosion of reinforcement in concrete construction
2145:
67:. Unsourced material may be challenged and removed.
1214:suspended vertically in the solution to act as an
1126:layer of oil, grease or a special mixture such as
1198:Rust removal from small iron or steel objects by
2288:
2052:
972:, and other electroactive conductive polymers.
1663:Ullmann's Encyclopedia of Industrial Chemistry
1635:
523:; it was continuously exposed to moisture and
599:in water. Under these corrosive conditions,
515:Heavy rust on the links of a chain near the
1830:"Cathodic Protection Systems - Matcor, Inc"
1113:blocks during a restoration attempt of the
948:. Similar passivation behavior occurs with
2198:
1888:
1579:
844:materials with the nominal formulae Fe(OH)
143:Colors and porous surface texture of rust
127:Learn how and when to remove this message
2120:International Journal of Hydrogen Energy
1609:
1607:
1580:Sund, Robert B.; Bishop, Jeanne (1980).
1284:
1078:
1068:
992:
931:
917:
894:
530:
510:
398:), and is typically associated with the
138:
2159:from the original on September 25, 2016
2113:
2031:Young Scientist Series ICSE Chemistry 7
616:
14:
2289:
2027:
1978:"Unlocking Mysteries of the Parthenon"
1046:
452:in an environment deprived of oxygen.
2205:(2 ed.). Boca Raton: CRC Press.
2028:Mirza, Lorraine; Gupta, Krishnakali.
1975:
1864:. Trenton Corporation. Archived from
1859:"Wax-Tape Anticorrosion Wrap Systems"
1604:
1434:
1297:showing the expansion of rusting iron
840:). High oxygen concentrations favour
713:affect the course of rust formation:
709:In addition, the following multistep
506:
2273:) Volume 1and 2; Editor: L L Shreir
1727:"Corrosion prevention - HomoFaciens"
1636:Holleman, A. F.; Wiberg, E. (2001).
1502:. Oxford University Press. June 2018
1309:Rust was an important factor in the
609:
604:the system are removed or consumed.
563:
65:adding citations to reliable sources
36:
2070:from the original on March 30, 2015
1171:
629:, as illustrated by the effects of
24:
2175:
2003:"Anti-seize Compounds Information"
1561:from the original on 9 August 2016
1351:Structural failures caused by rust
1272:
25:
2313:
2088:
2060:"Rust Removal using Electrolysis"
1724:
1425:concrete off the surface of this
2188:Simon & Schuster, New York.
2048:from the original on 2017-11-30.
1714:from the original on 2018-05-02.
1697:Ohmic Heating in Food Processing
1600:from the original on 2017-11-30.
1532:from the original on 2007-11-13.
1410:
1391:
1375:
1355:
982:
499:, which effectively fuelled the
155:
41:
2107:
2082:
2021:
1995:
1969:
1943:
1917:
1882:
1851:
1840:from the original on 2017-03-30
1822:
1794:
1766:
1748:
1737:from the original on 2017-12-01
1615:"Oxidation Reduction Reactions"
936:Cor-Ten sheet with rust coating
544:called rusting. Rusting is an
52:needs additional citations for
2199:Schweitzer, Philip A. (2007).
2132:10.1016/j.ijhydene.2022.11.219
1925:"Corrosion of Embedded Metals"
1892:Corrosion engineering handbook
1718:
1687:
1654:
1629:
1573:
1536:
1514:
1488:
591:Other degrading solutions are
13:
1:
1931:. Portland Cement Association
1889:Schweitzer, Philip A (2007).
1640:. San Diego: Academic Press.
1522:"Interview, David Des Marais"
1481:
1156:
890:
2153:"Rust Removal with Molasses"
1193:
7:
2034:. Pearson Education India.
1469:
885:ferroxyl indicator solution
572:is present, for example in
10:
2318:
1402:struts of the 70-year-old
1278:
1175:
1160:
1145:
1072:
1050:
986:
921:
482:
354:formed by the reaction of
350:, a usually reddish-brown
301:Other iron-based materials
29:
2267:Corrosion – 2nd Edition (
2114:Derwent, Richard (2023).
1895:. Boca Raton: CRC Press.
1141:
392:iron(III) oxide-hydroxide
1439:Rust is a commonly used
940:Stainless steel forms a
376:hydrous iron(III) oxides
237:Widmanstätten structures
2186:Rust – the longest war.
1671:10.1002/14356007.b01_08
1404:Nandu River Iron Bridge
425:is the common term for
2259:: CS1 maint: others (
1298:
1232:hydrogen embrittlement
1084:
998:
937:
903:
705:→ 4 Fe + 2 O
540:
528:
489:banded iron formations
429:of elemental iron and
144:
1617:. Bodner Research Web
1476:Corrosion engineering
1288:
1082:
1069:Coatings and painting
996:
935:
918:Rust-resistant alloys
898:
534:
514:
501:Industrial Revolution
142:
32:Rust (disambiguation)
1780:. AZoM. 28 June 2016
1451:, once dominated by
1329:was blown down by a
1313:disaster of 1967 in
752:as do the following
734:Fe + 3 H
717:Fe + 2 H
617:Associated reactions
421:on copper surfaces.
61:improve this article
2064:antique-engines.com
1760:Electrochimica Acta
1638:Inorganic Chemistry
1496:"Rust, n.1 and adj"
1457:automotive industry
1427:reinforced concrete
1366:, as seen from the
1337:Reinforced concrete
1304:Mianus river bridge
1163:Corrosion inhibitor
1060:electrode potential
1053:Cathodic protection
1047:Cathodic protection
1015:cathodic protection
1007:hot-dip galvanizing
946:chromium(III) oxide
798:2 FeO(OH) ⇌ Fe
711:acid–base reactions
456:used in underwater
374:. Rust consists of
232:Tempered martensite
1982:smithsonianmag.com
1774:"Weathering Steel"
1731:www.homofaciens.de
1449:American Northeast
1435:Cultural symbolism
1386:after it collapsed
1299:
1261:; or removed with
1085:
999:
938:
904:
613:for more details.
541:
529:
517:Golden Gate Bridge
507:Chemical reactions
463:, which generates
413:, and provides no
145:
27:Type of iron oxide
2212:978-0-8493-8244-4
2194:978-1-4516-9159-7
2126:(22): 8328–8341.
2009:. Engineering 360
1976:Hadingham, Evan.
1955:exploratorium.edu
1902:978-0-8493-8246-8
1583:Accent on science
1421:has expanded and
1319:suspension bridge
1259:hydrochloric acid
1030:sacrificial anode
683:Fe → Fe + 2
668:Because it forms
582:passivation layer
564:Oxidation of iron
341:
340:
137:
136:
129:
111:
16:(Redirected from
2309:
2264:
2258:
2250:
2224:
2169:
2168:
2166:
2164:
2149:
2143:
2142:
2140:
2138:
2111:
2105:
2104:
2102:
2101:
2086:
2080:
2079:
2077:
2075:
2056:
2050:
2049:
2025:
2019:
2018:
2016:
2014:
1999:
1993:
1992:
1990:
1988:
1973:
1967:
1966:
1964:
1962:
1947:
1941:
1940:
1938:
1936:
1921:
1915:
1914:
1886:
1880:
1879:
1877:
1876:
1870:
1863:
1855:
1849:
1848:
1846:
1845:
1826:
1820:
1819:
1814:
1813:
1798:
1792:
1791:
1786:
1785:
1770:
1764:
1763:
1752:
1746:
1745:
1743:
1742:
1725:Heinz, Norbert.
1722:
1716:
1715:
1691:
1685:
1684:
1658:
1652:
1651:
1633:
1627:
1626:
1624:
1622:
1611:
1602:
1601:
1586:. C.E. Merrill.
1577:
1571:
1570:
1568:
1566:
1560:
1554:. Parks Canada.
1549:
1540:
1534:
1533:
1518:
1512:
1511:
1509:
1507:
1492:
1445:American Midwest
1414:
1395:
1379:
1359:
1257:or the stronger
1172:Humidity control
977:weathering steel
928:Weathering steel
863:
862:
858:
815:
795:
776:
745:
728:
686:
661:
657:
644:
394:(FeO(OH), Fe(OH)
288:Weathering steel
283:High-speed steel
159:
147:
146:
132:
125:
121:
118:
112:
110:
69:
45:
37:
21:
2317:
2316:
2312:
2311:
2310:
2308:
2307:
2306:
2287:
2286:
2284:
2252:
2251:
2239:
2227:
2213:
2178:
2176:Further reading
2173:
2172:
2162:
2160:
2155:. 6 July 2005.
2151:
2150:
2146:
2136:
2134:
2112:
2108:
2099:
2097:
2087:
2083:
2073:
2071:
2058:
2057:
2053:
2042:
2026:
2022:
2012:
2010:
2001:
2000:
1996:
1986:
1984:
1974:
1970:
1960:
1958:
1957:. 17 April 2018
1949:
1948:
1944:
1934:
1932:
1923:
1922:
1918:
1903:
1887:
1883:
1874:
1872:
1868:
1861:
1857:
1856:
1852:
1843:
1841:
1828:
1827:
1823:
1811:
1809:
1806:greenspec.co.uk
1800:
1799:
1795:
1783:
1781:
1772:
1771:
1767:
1754:
1753:
1749:
1740:
1738:
1723:
1719:
1708:
1692:
1688:
1681:
1659:
1655:
1648:
1634:
1630:
1620:
1618:
1613:
1612:
1605:
1594:
1578:
1574:
1564:
1562:
1558:
1547:
1541:
1537:
1520:
1519:
1515:
1505:
1503:
1494:
1493:
1489:
1484:
1472:
1453:steel foundries
1437:
1430:
1415:
1406:
1396:
1387:
1380:
1371:
1360:
1347:and buildings.
1317:, when a steel
1283:
1275:
1273:Economic effect
1247:phosphoric acid
1224:battery charger
1196:
1184:
1174:
1165:
1159:
1150:
1144:
1077:
1071:
1055:
1049:
991:
985:
930:
924:Stainless steel
920:
893:
864:
860:
854:
853:
850:
839:
835:
812:
807:
805:
801:
792:
787:
785:
773:
768:
766:
743:
741:
737:
726:
724:
720:
704:
684:
659:
654:
649:
642:
640:
619:
610:economic effect
566:
509:
485:
473:aluminium oxide
397:
389:
385:
381:
278:Stainless steel
203:Microstructures
133:
122:
116:
113:
70:
68:
58:
46:
35:
28:
23:
22:
15:
12:
11:
5:
2315:
2305:
2304:
2299:
2282:
2281:
2265:
2237:
2225:
2211:
2196:
2177:
2174:
2171:
2170:
2144:
2106:
2081:
2051:
2040:
2020:
2007:globalspec.com
1994:
1968:
1942:
1916:
1901:
1881:
1850:
1821:
1793:
1765:
1747:
1717:
1706:
1686:
1679:
1653:
1646:
1628:
1603:
1592:
1572:
1535:
1513:
1486:
1485:
1483:
1480:
1479:
1478:
1471:
1468:
1436:
1433:
1432:
1431:
1416:
1409:
1407:
1397:
1390:
1388:
1381:
1374:
1372:
1362:The collapsed
1361:
1354:
1352:
1279:Main article:
1274:
1271:
1241:which contain
1239:rust converter
1210:, a length of
1195:
1192:
1173:
1170:
1161:Main article:
1158:
1155:
1148:Bluing (steel)
1146:Main article:
1143:
1140:
1119:Athens, Greece
1073:Main article:
1070:
1067:
1051:Main article:
1048:
1045:
1011:electroplating
987:Main article:
984:
981:
919:
916:
892:
889:
852:
845:
837:
833:
817:
816:
810:
803:
799:
796:
790:
783:
777:
771:
764:
750:
749:
739:
735:
732:
722:
718:
707:
706:
702:
695:redox reaction
693:The following
691:
690:
666:
665:
652:
638:
618:
615:
601:iron hydroxide
597:carbon dioxide
593:sulfur dioxide
565:
562:
508:
505:
484:
481:
395:
387:
383:
379:
339:
338:
337:
336:
331:
329:Malleable iron
326:
321:
316:
311:
303:
302:
298:
297:
296:
295:
290:
285:
280:
275:
273:Maraging steel
270:
265:
260:
255:
253:Crucible steel
247:
246:
242:
241:
240:
239:
234:
229:
224:
219:
214:
206:
205:
199:
198:
197:
196:
191:
186:
181:
176:
168:
167:
161:
160:
152:
151:
135:
134:
49:
47:
40:
26:
9:
6:
4:
3:
2:
2314:
2303:
2300:
2298:
2295:
2294:
2292:
2285:
2280:
2279:9781483164106
2276:
2272:
2271:
2266:
2262:
2256:
2248:
2244:
2240:
2238:0-85404-731-X
2234:
2230:
2226:
2222:
2218:
2214:
2208:
2204:
2203:
2197:
2195:
2191:
2187:
2183:
2180:
2179:
2158:
2154:
2148:
2133:
2129:
2125:
2121:
2117:
2110:
2096:
2092:
2089:Smith, Paul.
2085:
2069:
2065:
2061:
2055:
2047:
2043:
2041:9788131756591
2037:
2033:
2032:
2024:
2008:
2004:
1998:
1983:
1979:
1972:
1956:
1952:
1946:
1930:
1926:
1920:
1912:
1908:
1904:
1898:
1894:
1893:
1885:
1871:on 2018-03-23
1867:
1860:
1854:
1839:
1835:
1831:
1825:
1818:
1807:
1803:
1797:
1790:
1779:
1775:
1769:
1761:
1757:
1751:
1736:
1732:
1728:
1721:
1713:
1709:
1707:9781420071092
1703:
1700:. CRC Press.
1699:
1698:
1690:
1682:
1676:
1672:
1668:
1665:. Wiley-VCH.
1664:
1657:
1649:
1647:0-12-352651-5
1643:
1639:
1632:
1616:
1610:
1608:
1599:
1595:
1593:9780675075695
1589:
1585:
1584:
1576:
1557:
1553:
1546:
1539:
1531:
1527:
1523:
1517:
1501:
1497:
1491:
1487:
1477:
1474:
1473:
1467:
1464:
1462:
1458:
1454:
1450:
1446:
1442:
1428:
1424:
1420:
1413:
1408:
1405:
1401:
1394:
1389:
1385:
1384:Kinzua Bridge
1378:
1373:
1369:
1365:
1364:Silver Bridge
1358:
1353:
1350:
1349:
1348:
1346:
1342:
1338:
1334:
1332:
1328:
1324:
1323:Kinzua Bridge
1320:
1316:
1315:West Virginia
1312:
1311:Silver Bridge
1307:
1305:
1296:
1295:Exploratorium
1292:
1287:
1282:
1277:
1270:
1268:
1264:
1260:
1256:
1252:
1248:
1244:
1240:
1235:
1233:
1229:
1225:
1221:
1217:
1213:
1209:
1206:dissolved in
1205:
1201:
1191:
1189:
1183:
1179:
1169:
1164:
1154:
1149:
1139:
1135:
1133:
1129:
1124:
1120:
1116:
1112:
1107:
1105:
1102:
1098:
1094:
1090:
1081:
1076:
1066:
1063:
1061:
1054:
1044:
1042:
1037:
1035:
1031:
1027:
1022:
1020:
1016:
1012:
1008:
1004:
995:
990:
989:Galvanization
983:Galvanization
980:
978:
973:
971:
967:
963:
959:
955:
951:
947:
943:
934:
929:
925:
915:
913:
908:
901:
897:
888:
886:
881:
879:
875:
871:
866:
857:
849:
843:
831:
827:
823:
814:
797:
794:
781:
778:
775:
762:
759:
758:
757:
755:
748:
733:
731:
716:
715:
714:
712:
701:4 Fe + O
700:
699:
698:
696:
689:
682:
681:
680:
678:
674:
671:
664:
656:
647:
636:
635:
634:
632:
628:
624:
614:
612:
611:
605:
602:
598:
595:in water and
594:
589:
587:
586:ferrous oxide
583:
579:
575:
571:
561:
558:
554:
549:
547:
538:
533:
526:
522:
521:San Francisco
518:
513:
504:
502:
498:
494:
490:
480:
478:
474:
470:
466:
462:
459:
455:
451:
447:
442:
440:
437:. Many other
436:
432:
428:
424:
420:
416:
415:passivational
412:
407:
405:
401:
393:
377:
373:
369:
365:
361:
357:
353:
349:
345:
335:
332:
330:
327:
325:
322:
320:
317:
315:
312:
310:
307:
306:
305:
304:
300:
299:
294:
291:
289:
286:
284:
281:
279:
276:
274:
271:
269:
266:
264:
261:
259:
256:
254:
251:
250:
249:
248:
244:
243:
238:
235:
233:
230:
228:
225:
223:
220:
218:
215:
213:
210:
209:
208:
207:
204:
201:
200:
195:
192:
190:
187:
185:
182:
180:
177:
175:
172:
171:
170:
169:
166:
163:
162:
158:
154:
153:
149:
148:
141:
131:
128:
120:
109:
106:
102:
99:
95:
92:
88:
85:
81:
78: –
77:
73:
72:Find sources:
66:
62:
56:
55:
50:This article
48:
44:
39:
38:
33:
19:
2283:
2270:elsevier.com
2269:
2228:
2201:
2185:
2163:November 29,
2161:. Retrieved
2147:
2135:. Retrieved
2123:
2119:
2109:
2098:. Retrieved
2094:
2084:
2072:. Retrieved
2063:
2054:
2030:
2023:
2011:. Retrieved
2006:
1997:
1985:. Retrieved
1981:
1971:
1959:. Retrieved
1954:
1945:
1933:. Retrieved
1928:
1919:
1891:
1884:
1873:. Retrieved
1866:the original
1853:
1842:. Retrieved
1833:
1824:
1816:
1810:. Retrieved
1805:
1796:
1788:
1782:. Retrieved
1777:
1768:
1759:
1750:
1739:. Retrieved
1730:
1720:
1696:
1689:
1662:
1656:
1637:
1631:
1619:. Retrieved
1582:
1575:
1563:. Retrieved
1551:
1538:
1525:
1516:
1504:. Retrieved
1499:
1490:
1465:
1438:
1335:
1327:Pennsylvania
1308:
1300:
1290:
1276:
1236:
1204:washing soda
1200:electrolysis
1197:
1185:
1178:Dehumidifier
1166:
1151:
1136:
1108:
1086:
1075:Rustproofing
1064:
1056:
1038:
1025:
1023:
1000:
974:
939:
909:
905:
882:
867:
855:
847:
818:
786:⇌ FeO(OH) +
756:equilibria:
751:
708:
692:
687:
667:
645:
627:electrolytes
620:
608:
606:
590:
567:
550:
542:
486:
468:
446:spectroscopy
443:
422:
408:
404:refined iron
372:air moisture
366:presence of
343:
342:
334:Wrought iron
324:Ductile iron
263:Spring steel
258:Carbon steel
123:
114:
104:
97:
90:
83:
71:
59:Please help
54:verification
51:
2182:Waldman, J.
2137:December 9,
1834:Matcor, Inc
1808:. greenspec
1552:depotwijzer
1398:Rusted and
1251:citric acid
1243:tannic acid
1220:baling wire
1132:mothballing
970:polyaniline
962:zinc oxides
942:passivation
754:dehydration
469:stable rust
268:Alloy steel
212:Spheroidite
2291:Categories
2100:2023-12-29
1929:cement.org
1875:2018-03-22
1844:2017-03-29
1812:2022-09-13
1784:2022-09-13
1741:2017-11-30
1680:3527306730
1500:OED Online
1482:References
1291:Rust Wedge
1188:silica gel
1176:See also:
1157:Inhibitors
1026:zinc-alume
1005:by either
922:See also:
891:Prevention
874:hydroxides
824:and black
738:O ⇌ Fe(OH)
721:O ⇌ Fe(OH)
578:salt spray
525:salt spray
465:green rust
431:its alloys
348:iron oxide
319:White iron
293:Tool steel
227:Ledeburite
189:Martensite
87:newspapers
2297:Corrosion
2255:cite book
2221:137248972
1911:137248977
1461:Rust Belt
1281:Corrosion
1263:chelating
1208:tap water
1194:Treatment
1182:Desiccant
1128:Cosmoline
1115:Parthenon
975:Special "
966:aluminium
950:magnesium
944:layer of
912:permeable
830:magnetite
826:lodestone
742:+ 3
725:+ 2
670:hydroxide
658:→ 4
648:+ 2
641:+ 4
631:road salt
623:electrons
551:The main
546:oxidation
477:aluminium
427:corrosion
400:corrosion
364:catalytic
314:Gray iron
309:Cast iron
184:Cementite
179:Austenite
117:June 2012
2247:35233292
2184:(2015):
2157:Archived
2074:April 1,
2068:Archived
2046:Archived
1838:Archived
1778:azom.com
1735:Archived
1712:Archived
1621:28 April
1598:Archived
1556:Archived
1530:Archived
1528:. 2003.
1470:See also
1441:metaphor
1417:Rusting
1289:Outdoor
1267:molasses
1101:alkaline
954:titanium
910:Rust is
767:⇌ FeO +
574:seawater
553:catalyst
458:concrete
450:chloride
433:such as
217:Pearlite
194:Graphite
2095:Youtube
1565:23 July
1429:support
1423:spalled
1345:bridges
1331:tornado
1293:at the
1255:vinegar
1228:cathode
1097:varnish
1093:lacquer
1034:coating
1019:cadmium
900:Cor-Ten
859:⁄
744:
727:
685:
660:
643:
537:forging
483:History
461:pillars
423:Rusting
411:friable
390:O) and
362:in the
245:Classes
222:Bainite
174:Ferrite
101:scholar
18:Rusting
2277:
2245:
2235:
2219:
2209:
2192:
2038:
2013:9 July
1987:9 July
1961:9 July
1935:9 July
1909:
1899:
1704:
1677:
1644:
1590:
1506:7 July
1455:, the
1400:pitted
1142:Bluing
1111:marble
878:oxides
842:ferric
439:metals
419:patina
360:oxygen
346:is an
165:Phases
150:Steels
103:
96:
89:
82:
76:"Rust"
74:
1869:(PDF)
1862:(PDF)
1559:(PDF)
1548:(PDF)
1419:rebar
1341:spall
1216:anode
1212:rebar
1089:paint
497:steel
454:Rebar
435:steel
368:water
352:oxide
108:JSTOR
94:books
2302:Iron
2275:ISBN
2261:link
2243:OCLC
2233:ISBN
2217:OCLC
2207:ISBN
2190:ISBN
2165:2017
2139:2023
2076:2015
2036:ISBN
2015:2021
1989:2021
1963:2021
1937:2021
1907:OCLC
1897:ISBN
1702:ISBN
1675:ISBN
1642:ISBN
1623:2020
1588:ISBN
1567:2016
1526:NASA
1508:2018
1447:and
1382:The
1370:side
1368:Ohio
1253:and
1180:and
1123:lead
1003:zinc
958:zinc
926:and
876:and
782:(OH)
763:(OH)
673:ions
570:salt
557:pits
495:and
493:iron
358:and
356:iron
344:Rust
80:news
2128:doi
1667:doi
1463:".
1325:in
1245:or
1117:in
1009:or
832:(Fe
828:or
822:FeO
576:or
519:in
475:on
402:of
386:·nH
378:(Fe
370:or
63:by
2293::
2257:}}
2253:{{
2241:.
2215:.
2124:48
2122:.
2118:.
2093:.
2066:.
2062:.
2044:.
2005:.
1980:.
1953:.
1927:.
1905:.
1836:.
1832:.
1815:.
1804:.
1787:.
1776:.
1758:.
1733:.
1729:.
1710:.
1673:.
1606:^
1596:.
1550:.
1524:.
1498:.
1269:.
1104:pH
1095:,
1091:,
1041:μm
968:,
964:,
960:,
956:,
952:,
870:Ca
846:3−
806:+
780:Fe
761:Fe
677:pH
663:OH
503:.
479:.
406:.
2263:)
2249:.
2223:.
2167:.
2141:.
2130::
2103:.
2078:.
2017:.
1991:.
1965:.
1939:.
1913:.
1878:.
1847:.
1762:.
1744:.
1683:.
1669::
1650:.
1625:.
1569:.
1510:.
861:2
856:x
851:O
848:x
838:4
836:O
834:3
813:O
811:2
809:H
804:3
802:O
800:2
793:O
791:2
789:H
784:3
774:O
772:2
770:H
765:2
747:H
740:3
736:2
730:H
723:2
719:2
703:2
688:e
655:O
653:2
651:H
646:e
639:2
637:O
396:3
388:2
384:3
382:O
380:2
130:)
124:(
119:)
115:(
105:·
98:·
91:·
84:·
57:.
34:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.