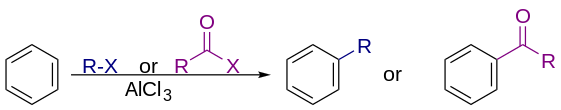469:
560:
789:
902:
200:
533:
1117:
394:
516:
921:
1145:
862:
1182:
4415:
387:
767:, a stoichiometric amount or more of the "catalyst" must generally be employed, unlike the case of the Friedel–Crafts alkylation, in which the catalyst is constantly regenerated. Reaction conditions are similar to the Friedel–Crafts alkylation. This reaction has several advantages over the alkylation reaction. Due to the electron-withdrawing effect of the
1014:
843:
catalyst. However, in contrast to the truly catalytic alkylation reaction, the formed ketone is a moderate Lewis base, which forms a complex with the strong Lewis acid aluminum trichloride. The formation of this complex is typically irreversible under reaction conditions. Thus, a stochiometric
479:
alkylating agents, some secondary alkylating agents (ones for which carbocation rearrangement is degenerate), or alkylating agents that yield stabilized carbocations (e.g., benzylic or allylic ones). In the case of primary alkyl halides, the carbocation-like complex
475:
Furthermore, the reaction is only useful for primary alkyl halides in an intramolecular sense when a 5- or 6-membered ring is formed. For the intermolecular case, the reaction is limited to
5389:
795:
The viability of the
Friedel–Crafts acylation depends on the stability of the acyl chloride reagent. Formyl chloride, for example, is too unstable to be isolated. Thus, synthesis of
852:
with respect to the limiting reagent, phenylacetyl chloride. In certain cases, generally when the benzene ring is activated, Friedel–Crafts acylation can also be carried out with
848:
is needed. The complex is destroyed upon aqueous workup to give the desired ketone. For example, the classical synthesis of deoxybenzoin calls for 1.1 equivalents of AlCl
7604:
2043:"Optically active .alpha.- and .beta.-naphthalene derivatives. 5. Stereochemical course of the Haworth-type synthesis of optically active 2-(1-methylpropyl)naphthalene"
539:
For primary (and possibly secondary) alkyl halides, a carbocation-like complex with the Lewis acid, is more likely to be involved, rather than a free carbocation.
2387:
3078:
3068:
3063:
856:
amounts of a milder Lewis acid (e.g. Zn(II) salts) or a Brønsted acid catalyst using the anhydride or even the carboxylic acid itself as the acylation agent.
3777:
3320:
3088:
3083:
3073:
3006:
2996:
2990:
3335:
3325:
3058:
3048:
3016:
3011:
3001:
3762:
3213:
3053:
2985:
2974:
1211:
catalyst gives triarylmethanes, which are often brightly colored, as is the case in triarylmethane dyes. This is a bench test for aromatic compounds.
6720:
6665:
3043:
2079:
John C. Gilbert., Stephen F. Martin. Brooks/Cole CENGAGE Learning, 2011. pp 872. 25.10 Aromatic
Hydrocarbons and Aryl Halides – Classification test.
7433:
4016:
3757:
2919:
1500:
6775:
6925:
5559:
4021:
4011:
2908:
1496:
373:, some of the largest scale reactions practiced in industry. Such alkylations are of major industrial importance, e.g. for the production of
7758:
7654:
730:
463:-butylation of 1,4-dimethoxybenzene that gives only the product of two alkylation cycles and with only one of three possible isomers of it:
351:
151:
7428:
5254:
1842:
Grzybowski, M.; Skonieczny, K.; Butenschön, H.; Gryko, D. T. (2013). "Comparison of
Oxidative Aromatic Coupling and the Scholl Reaction".
6530:
4451:
468:
7100:
6300:
5044:
823:. Simple ketones that could be obtained by Friedel–Crafts acylation are produced by alternative methods, e.g., oxidation, in industry.
452:
7265:
7195:
7175:
6670:
5837:
5299:
5718:
5274:
393:
4487:
17:
7020:
7498:
7448:
5852:
6955:
788:
7594:
7403:
7060:
7040:
7000:
5807:
4750:
2084:
1790:
1532:
1398:
1361:
7589:
7519:
7418:
7075:
6930:
6560:
6405:
6015:
5642:
5419:
1575:
Tsai, Tseng-Chang "Disproportionation and
Transalkylation of Alkylbenzenes over Zeolite Catalysts". Elsevier Science, 1999
1188:
The product formed in this reaction is then analogously reduced, followed by a dehydrogenation reaction (with the reagent
7669:
7453:
6765:
6255:
5930:
1040:
193:
6475:
901:
7664:
7378:
7240:
7030:
6995:
2363:
377:, the precursor to polystyrene, from benzene and ethylene and for the production of cumene from benzene and propene in
7554:
7493:
7025:
6940:
6910:
6890:
6755:
6750:
6125:
6050:
5693:
5647:
5514:
4775:
4366:
4166:
2028:
1814:
1472:
1249:
Friedel, C.; Crafts, J. M. (1877) "Sur une nouvelle méthode générale de synthèse d'hydrocarbures, d'acétones, etc.,"
775:
product is always less reactive than the original molecule, so multiple acylations do not occur. Also, there are no
7659:
7619:
7569:
7245:
7045:
6795:
6725:
5214:
4785:
1620:
6860:
5753:
5474:
831:
The reaction proceeds through generation of an acylium center. The reaction is completed by deprotonation of the
336:
7753:
7413:
7255:
7125:
7120:
6935:
6410:
6320:
5910:
5842:
5733:
5309:
5064:
4989:
4444:
4395:
1698:
715:
7170:
1942:
Sereda, Grigoriy A.; Rajpara, Vikul B. (2007). "A Green
Alternative to Aluminum Chloride Alkylation of Xylene".
7699:
7584:
7483:
7423:
7070:
6865:
6825:
6800:
6710:
6170:
5204:
5134:
4770:
4700:
1602:
1296:
868:
If desired, the resulting ketone can be subsequently reduced to the corresponding alkane substituent by either
6290:
1416:"A review of new developments in the Friedel–Crafts alkylation – From green chemistry to asymmetric catalysis"
7689:
7275:
7165:
6785:
6510:
6295:
6240:
6085:
6045:
5877:
5632:
5349:
5199:
4261:
3355:
3298:
1116:
7649:
7210:
6655:
7684:
7599:
7574:
7549:
7534:
7458:
7373:
7270:
7230:
7095:
7050:
6815:
6360:
6345:
6210:
6000:
5668:
5424:
5104:
5079:
5049:
4640:
944:
7748:
7634:
7579:
7524:
7235:
7155:
7055:
6770:
6735:
6580:
6470:
6185:
6180:
6005:
5965:
5862:
5673:
5637:
5489:
5479:
5334:
5054:
5004:
4999:
4974:
4934:
4880:
4645:
4635:
4610:
4309:
2661:
2533:
1780:
1522:
1173:
876:. The net result is the same as the Friedel–Crafts alkylation except that rearrangement is not possible.
869:
1079:. This variation will not work with primary halides from which less carbocation involvement is inferred.
7609:
7310:
7115:
6550:
6435:
6115:
6090:
6030:
5887:
5622:
5329:
5109:
5074:
4979:
4670:
4605:
4437:
4371:
3670:
2601:
2596:
958:
723:
344:
144:
6900:
5164:
7709:
7614:
7468:
7348:
7320:
7290:
7205:
7135:
7090:
7065:
6985:
6885:
6845:
6540:
6160:
6150:
6075:
5599:
5459:
5454:
5434:
5119:
4916:
4895:
4855:
4780:
4390:
4381:
4361:
2591:
4600:
1144:
491:
to give almost exclusively the rearranged product derived from a secondary or tertiary carbocation.
7674:
7564:
7544:
7408:
7250:
7160:
7130:
7110:
7005:
6960:
6790:
6700:
6630:
6515:
6505:
6335:
5892:
5832:
5797:
5604:
5584:
5544:
5319:
5189:
5154:
5114:
4865:
4705:
4695:
4625:
3715:
1980:"Synthesis of Triarylmethane and Xanthene Dyes Using Electrophilic Aromatic Substitution Reactions"
804:
763:. Because, however, the product ketone forms a rather stable complex with Lewis acids such as AlCl
7140:
7763:
7644:
7503:
7353:
7295:
7220:
7200:
6920:
6870:
6730:
6695:
6635:
6565:
6120:
5867:
5847:
5579:
5499:
5394:
5354:
5324:
5259:
5144:
5129:
5039:
5029:
4690:
4615:
4570:
4246:
3612:
986:
a chloromethyl group is added to an arene with formaldehyde, hydrochloric acid and zinc chloride.
983:
937:
579:
4905:
1585:
Helmut Fiege; Heinz-Werner Voges; Toshikazu
Hamamoto; et al. (2002). "Phenol Derivatives".
1160:
is a classic method for the synthesis of polycyclic aromatic hydrocarbons. In this reaction, an
924:
Alkylation of benzene & ethylene, one of the largest scale reactions practiced commercially.
7383:
7105:
6855:
6835:
6810:
6760:
6675:
6650:
6605:
6575:
6555:
6525:
6490:
6445:
6420:
6395:
6280:
6205:
5985:
5678:
5614:
5414:
5139:
5059:
4745:
4720:
4497:
4492:
4251:
2636:
2586:
2581:
2356:
1161:
563:
488:
2018:
7719:
7305:
7260:
6975:
6945:
6915:
6850:
6830:
6745:
6740:
6705:
6660:
6645:
6640:
6620:
6610:
6545:
6535:
6465:
6415:
5935:
5738:
5314:
5269:
5099:
5089:
4835:
4760:
4555:
4517:
4286:
3675:
2318:
2295:
2272:
2249:
2226:
2203:
2180:
2152:
2129:
2106:
1584:
760:
559:
4765:
1181:
7488:
7438:
7388:
7368:
7358:
7215:
7190:
6905:
6895:
6780:
6595:
6590:
6520:
6305:
6105:
6065:
5995:
5960:
5915:
5882:
5748:
5723:
5703:
5524:
5484:
5444:
5409:
5339:
5094:
4964:
4939:
4477:
4376:
2673:
2646:
1992:
1952:
1877:
1169:
994:
933:
873:
459:
can be exploited to limit the number of successive alkylation cycles that occur, as in the
181:
1275:
Price, C. C. (1946). "The
Alkylation of Aromatic Compounds by the Friedel-Crafts Method".
1262:
1258:
8:
7694:
7679:
7325:
7300:
7285:
7280:
7010:
6965:
6950:
6840:
6820:
6715:
6600:
6585:
6430:
6375:
6365:
6330:
6095:
5970:
5945:
5857:
5713:
5698:
5683:
5504:
5449:
5219:
5069:
5014:
4885:
4800:
4660:
4585:
4400:
3752:
3695:
3657:
3330:
3315:
3293:
2641:
2553:
2453:
2372:
965:
552:. Although this is usually undesirable it can be exploited; for instance by facilitating
549:
7704:
6355:
5539:
4730:
1996:
1956:
7443:
7393:
7363:
7225:
7015:
6805:
6690:
6625:
6615:
6380:
6310:
6275:
6270:
6250:
6245:
6190:
6100:
5950:
5812:
5802:
5708:
5494:
5439:
5369:
5289:
5184:
5084:
5019:
4944:
4790:
4655:
4590:
4575:
4314:
4295:
4171:
3787:
3632:
3627:
3622:
3617:
3372:
3238:
2704:
2543:
2435:
2402:
2250:"Preparation of 9,10-dimethoxyphenanthrene and 3,6-diacetyl-9,10-dimethoxyphenanthrene"
1490:
1440:
1415:
1390:
1353:
1208:
1165:
1108:
1061:
951:
816:
503:
441:
1053:(1881) is the ring acetylation of phenols with acids in the presence of zinc chloride.
888:
and ketones to form the hydroxyalkylated products, for example in the reaction of the
783:
is stabilized by a resonance structure in which the positive charge is on the oxygen.
7180:
6500:
6385:
6350:
6315:
6260:
6215:
6130:
6110:
6060:
6055:
6025:
6010:
5920:
5827:
5763:
5728:
5554:
5429:
5304:
5229:
5209:
5124:
4959:
4954:
4900:
4810:
4715:
4675:
4630:
4512:
4507:
4472:
4418:
4349:
4270:
3782:
3385:
3364:
3340:
3288:
3283:
3186:
3166:
3156:
3146:
3141:
2626:
2558:
2494:
2427:
2422:
2349:
2326:
2303:
2280:
2257:
2234:
2211:
2188:
2160:
2137:
2114:
2080:
2062:
2024:
1859:
1810:
1786:
1725:
1711:
Fuson, R. C.; Weinstock, H. H.; Ullyot, G. E. (1935). "A New
Synthesis of Benzoins. 2
1598:
1549:
1528:
1478:
1468:
1445:
1394:
1357:
1292:
1226:
1157:
812:
695:
615:
316:
236:
128:
57:
6175:
4290:
7714:
7559:
7529:
7473:
7398:
7330:
7085:
7035:
6880:
6685:
6460:
6455:
6400:
6390:
6165:
5975:
5955:
5925:
5822:
5758:
5743:
5574:
5529:
5519:
5509:
5404:
5384:
5379:
5364:
5359:
5239:
5234:
5174:
5159:
5149:
4994:
4984:
4850:
4840:
4740:
4735:
4710:
4650:
4502:
4461:
3819:
3767:
3710:
3537:
3484:
3380:
3278:
3181:
3151:
3131:
3121:
2979:
2631:
2054:
2042:
2000:
1960:
1924:
1891:
1851:
1733:
1656:
1629:
1590:
1558:
1435:
1427:
1386:
1349:
1322:
1284:
1189:
1089:
820:
575:
571:
499:
456:
199:
169:
4830:
1979:
964:
A reaction modification with an aromatic phenyl ester as a reactant is called the
7624:
7315:
7150:
7145:
6440:
6425:
6370:
6325:
6285:
6235:
6200:
6195:
6140:
6135:
6070:
6020:
5940:
5768:
5652:
5627:
5589:
5564:
5549:
5534:
5469:
5344:
5294:
5284:
5264:
5224:
5034:
5024:
5009:
4805:
4725:
4595:
4565:
4550:
4545:
4354:
4323:
4256:
4223:
4176:
4161:
3829:
3523:
3518:
3479:
3203:
3171:
3136:
2686:
2621:
2616:
2611:
2606:
2548:
2513:
2412:
1915:
1314:
1231:
1100:
1057:
1029:
972:
808:
601:
553:
476:
222:
173:
43:
1882:
Kamp, J. V. D.; Mosettig, E. (1936). "Trans- and Cis-As-Octahydrophenanthrene".
1674:
1288:
932:
The acylated reaction product can be converted into the alkylated product via a
799:
through the
Friedel–Crafts pathway requires that formyl chloride be synthesized
7629:
7539:
7478:
6570:
6480:
6450:
6225:
6080:
5817:
5594:
5464:
5279:
5249:
4949:
4845:
4620:
4482:
4385:
4300:
4156:
3705:
3665:
3350:
3126:
3116:
3111:
3101:
2968:
2930:
2913:
2897:
2853:
2842:
2836:
2681:
2576:
2466:
1984:
1944:
1220:
1176:. Lastly, a second Friedel-Crafts acylation takes place with addition of acid.
752:
532:
378:
4429:
7742:
7639:
7340:
7185:
7080:
6875:
6265:
6230:
6220:
6155:
6145:
6035:
5872:
5688:
5399:
5374:
5244:
4890:
4875:
4860:
4755:
4685:
4665:
4580:
4339:
4238:
3797:
3772:
3744:
3687:
3510:
3458:
3345:
3273:
3268:
3106:
2941:
2935:
2924:
2864:
2858:
2847:
2538:
2486:
2461:
2066:
1928:
1660:
1594:
1562:
1482:
1128:
1076:
998:
748:
566:
is produced via transalkylation, a special form of
Friedel–Crafts alkylation.
515:
507:
428:. Many alkylating agents can be used instead of alkyl halides. For example,
421:
920:
436:
can be used in presence of protons. The reaction typically employs a strong
6680:
6040:
5792:
5569:
5169:
4969:
4820:
4815:
4680:
4535:
4228:
3604:
3263:
2831:
2656:
1911:"Ueber die Verbindungen der ein- und zweibasischen Fettsäuren mit Phenolen"
1863:
1855:
1449:
1006:
796:
605:
425:
374:
226:
177:
47:
2092:
861:
5179:
4825:
4795:
4560:
4344:
4318:
4208:
4055:
3647:
3642:
3637:
3428:
2902:
2825:
2819:
1776:
1710:
1518:
1326:
1136:
1132:
1043:
1025:
832:
780:
776:
632:
495:
485:
448:
253:
2247:
2058:
1895:
1737:
1633:
1135:. Replacing resorcinol by N,N-diethylaminophenol in this reaction gives
1013:
7463:
6990:
6340:
4305:
4218:
4213:
4118:
3919:
3849:
3725:
3547:
3448:
3258:
3253:
3223:
3218:
3176:
2891:
2774:
2764:
2754:
2709:
2443:
2417:
2397:
2004:
1377:
Heaney, H. (1991). "The Bimolecular Aromatic Friedel–Crafts Reaction".
1204:
1124:
756:
444:
as catalyst, to increase the electrophilicity of the alkylating agent.
437:
417:
401:
264:
185:
1964:
1647:
Somerville, L. F.; Allen, C. F. H. (1933). "β-Benzoylpropionic acid".
4113:
4103:
4085:
3939:
3730:
3585:
3438:
3248:
3243:
3233:
3228:
3208:
2813:
2785:
2779:
2769:
2759:
2714:
1431:
744:
643:
498:, the electrophiles. A laboratory-scale example by the synthesis of
447:
This reaction suffers from the disadvantage that the product is more
386:
189:
1941:
1910:
1088:
Friedel–Crafts reactions have been used in the synthesis of several
908:
As usual, the aldehyde group is more reactive electrophile than the
4870:
4540:
4192:
4108:
4080:
4065:
4060:
4001:
3996:
3980:
3909:
3899:
3869:
3859:
3839:
3575:
3571:
3551:
3533:
3500:
3408:
2744:
2525:
2499:
1841:
1093:
1069:
1065:
885:
768:
683:
304:
116:
74:
1046:
is added making it a reductive acylation to methylcyclohexylketone
4530:
4070:
4050:
4006:
3962:
3889:
3879:
3720:
3700:
3561:
3418:
2749:
2696:
2651:
2568:
2474:
2407:
1312:
Groves, J. K. (1972). "The Friedel–Crafts acylation of alkenes".
909:
893:
889:
691:
433:
405:
312:
124:
1782:
Advanced Organic Chemistry: Reactions, Mechanisms, and Structure
1524:
Advanced Organic Chemistry: Reactions, Mechanisms, and Structure
1198:
527:
The general mechanism for primary alkyl halides is shown below.
369:
In commercial applications, the alkylating agents are generally
4136:
3929:
3161:
2319:"Carboxylation of aromatic compounds: ferrocenecarboxylic acid"
1104:
772:
370:
2104:
1340:
Eyley, S. C. (1991). "The Aliphatic Friedel–Crafts Reaction".
4075:
1774:
1546:
1516:
1467:. Masters, Katherine M. (Seventh ed.). Boston, MA, USA.
928:
This reaction is related to several classic named reactions:
429:
2020:
Name Reactions: A Collection of Detailed Reaction Mechanisms
1547:
Smith, W. T. Jr.; Sellas, J. T. (1952). "Neophyl Chloride".
1413:
2341:
1785:(6th ed.), New York: Wiley-Interscience, p. 725,
27:
Set of reactions to attach substituents to an aromatic ring
2224:
1744:
578:
via the oxidative coupling and subsequent dealkylation of
1823:
1756:
1096:
574:. This approach is used industrially in the synthesis of
2150:
2270:
755:
as well as carboxylic acids are also viable. A typical
570:
It also allows alkyl chains to be added reversibly as
2248:
Kamil Paruch; Libor Vyklicky; Thomas J. Katz (2003).
1168:, the subsequent product is then reduced in either a
7605:
Erlenmeyer–Plöchl azlactone and amino-acid synthesis
1908:
947:
can be used to synthesize benzaldehyde from benzene.
455:. Consequently, overalkylation can occur. However,
184:. Friedel–Crafts reactions are of two main types:
6666:Divinylcyclopropane-cycloheptadiene rearrangement
1195:for example) to extend the aromatic ring system.
7740:
2040:
1977:
954:describes arene reactions with hydrocyanic acid.
747:of aromatic rings. Typical acylating agents are
4459:
2316:
2127:
1978:McCullagh, James V.; Daggett, Kelly A. (2007).
1646:
815:under high pressure, catalyzed by a mixture of
6926:Thermal rearrangement of aromatic hydrocarbons
5560:Thermal rearrangement of aromatic hydrocarbons
2041:Menicagli, Rita; Piccolo, Oreste (June 1980).
1701:. Organic-chemistry.org. Retrieved 2014-01-11.
1587:Ullmann's Encyclopedia of Industrial Chemistry
1527:(6th ed.), New York: Wiley-Interscience,
543:
7655:Lectka enantioselective beta-lactam synthesis
4915:
4445:
2357:
1465:Macroscale and microscale organic experiments
1311:
1199:Friedel–Crafts test for aromatic hydrocarbons
975:two arenes couple directly (sometimes called
7434:Inverse electron-demand Diels–Alder reaction
5255:Heterogeneous metal catalyzed cross-coupling
2293:
2201:
1881:
1339:
1274:
424:. Traditionally, the alkylating agents are
198:
6776:Lobry de Bruyn–Van Ekenstein transformation
1512:
1510:
1499:) CS1 maint: multiple names: authors list (
451:than the reactant because alkyl groups are
4452:
4438:
2364:
2350:
2178:
1835:
1829:
1804:
1762:
1750:
1495:: CS1 maint: location missing publisher (
1463:L., Williamson, Kenneth (4 January 2016).
1376:
860:
453:activators for the Friedel–Crafts reaction
7266:Petrenko-Kritschenko piperidone synthesis
6721:Fritsch–Buttenberg–Wiechell rearrangement
2105:Everett M. Schultz; Sally Mickey (1949).
1439:
1103:(a pH indicator) from two equivalents of
7429:Intramolecular Diels–Alder cycloaddition
1884:Journal of the American Chemical Society
1618:Hay, Allan S. (1969). "p,p'-Biphenols".
1507:
961:describes arene reactions with nitriles.
919:
807:, accomplished by treating benzene with
661:Friedel-Crafts aromatic addition product
626:
558:
531:
282:Friedel-Crafts aromatic addition product
247:
68:
1414:Rueping, M.; Nachtsheim, B. J. (2010).
1024:(1910, 1936) involves the acylation of
1022:Darzens–Nenitzescu synthesis of ketones
915:
536:Mechanism of Friedel–Crafts alkylation.
416:Friedel–Crafts alkylation involves the
85:Alkyl Halide, Alcohol, Alkene or Alkyne
14:
7741:
7449:Metal-centered cycloaddition reactions
7101:Debus–Radziszewski imidazole synthesis
5045:Bodroux–Chichibabin aldehyde synthesis
2093:Friedel–Crafts reactions published on
1123:A reaction of phthalic anhydride with
743:Friedel–Crafts acylation involves the
7595:Diazoalkane 1,3-dipolar cycloaddition
7499:Vinylcyclopropane (5+2) cycloaddition
7404:Diazoalkane 1,3-dipolar cycloaddition
7176:Hurd–Mori 1,2,3-thiadiazole synthesis
6671:Dowd–Beckwith ring-expansion reaction
5838:Hurd–Mori 1,2,3-thiadiazole synthesis
4914:
4751:LFER solvent coefficients (data page)
4433:
2345:
2225:C. F. H. Allen; W. E. Barker (1932).
1768:
826:
411:
400:Industrial production typically uses
180:in 1877 to attach substituents to an
7759:Carbon-carbon bond forming reactions
6406:Sharpless asymmetric dihydroxylation
5643:Methoxymethylenetriphenylphosphorane
3511:Polyhydric alcohols (sugar alcohols)
1569:
1151:
879:
6531:Allen–Millar–Trippett rearrangement
2151:C. S. Marvel; W. M. Sperry (1928).
1617:
194:electrophilic aromatic substitution
24:
7670:Nitrone-olefin (3+2) cycloaddition
7665:Niementowski quinazoline synthesis
7454:Nitrone-olefin (3+2) cycloaddition
7379:Azide-alkyne Huisgen cycloaddition
7241:Niementowski quinazoline synthesis
6996:Azide-alkyne Huisgen cycloaddition
6301:Meerwein–Ponndorf–Verley reduction
5853:Leimgruber–Batcho indole synthesis
2271:Roger Adams; C. R. Noller (1925).
2204:"β-(3-Acenaphthoyl)Propionic acid"
1807:March's Advanced Organic Chemistry
1462:
1391:10.1016/B978-0-08-052349-1.00046-9
1354:10.1016/B978-0-08-052349-1.00045-7
548:Friedel–Crafts alkylations can be
25:
7775:
7494:Trimethylenemethane cycloaddition
7196:Johnson–Corey–Chaykovsky reaction
7061:Cadogan–Sundberg indole synthesis
7041:Bohlmann–Rahtz pyridine synthesis
7001:Baeyer–Emmerling indole synthesis
5808:Cadogan–Sundberg indole synthesis
5300:Johnson–Corey–Chaykovsky reaction
4167:2-Methyl-2-propyl-1,3-propanediol
1207:with aromatic compounds using an
494:Protonation of alkenes generates
7590:Cook–Heilbron thiazole synthesis
7419:Hexadehydro Diels–Alder reaction
7246:Niementowski quinoline synthesis
7076:Cook–Heilbron thiazole synthesis
7021:Bischler–Möhlau indole synthesis
6931:Tiffeneau–Demjanov rearrangement
6561:Baker–Venkataraman rearrangement
5719:Horner–Wadsworth–Emmons reaction
5390:Mizoroki-Heck vs. Reductive Heck
5275:Horner–Wadsworth–Emmons reaction
4786:Neighbouring group participation
4414:
4413:
2047:The Journal of Organic Chemistry
1621:The Journal of Organic Chemistry
1180:
1143:
1115:
1099:. Examples are the synthesis of
1012:
900:
787:
514:
467:
392:
385:
7126:Fiesselmann thiophene synthesis
6956:Westphalen–Lettré rearrangement
6936:Vinylcyclopropane rearrangement
6766:Kornblum–DeLaMare rearrangement
6411:Epoxidation of allylic alcohols
6321:Noyori asymmetric hydrogenation
6256:Kornblum–DeLaMare rearrangement
5931:Gallagher–Hollander degradation
4396:Nucleophilic conjugate addition
2073:
2034:
2011:
1971:
1935:
1909:Nencki, M.; Sieber, N. (1881).
1902:
1870:
1798:
1704:
1692:
1667:
1640:
1611:
1578:
1005:to the octahydro derivative of
364:
7585:Chichibabin pyridine synthesis
7071:Chichibabin pyridine synthesis
7031:Blum–Ittah aziridine synthesis
6866:Ring expansion and contraction
5135:Cross dehydrogenative coupling
1805:Smith, M.B.; March, J (2001).
1540:
1456:
1407:
1370:
1333:
1305:
1268:
1243:
1223:, lineage of French scientists
1037:Nenitzescu reductive acylation
803:. This is accomplished by the
669:HCl (reaction type dependent)
290:HCl (reaction type dependent)
13:
1:
7555:Bischler–Napieralski reaction
7513:Heterocycle forming reactions
7166:Hemetsberger indole synthesis
7026:Bischler–Napieralski reaction
6941:Wagner–Meerwein rearrangement
6911:Sommelet–Hauser rearrangement
6891:Seyferth–Gilbert homologation
6756:Ireland–Claisen rearrangement
6751:Hofmann–Martius rearrangement
6511:2,3-sigmatropic rearrangement
6126:Corey–Winter olefin synthesis
6051:Barton–McCombie deoxygenation
5694:Corey–Winter olefin synthesis
5648:Seyferth–Gilbert homologation
5515:Seyferth–Gilbert homologation
4367:Fischer–Speier esterification
3299:Propylene glycol methyl ether
1237:
206:
7660:Lehmstedt–Tanasescu reaction
7620:Gabriel–Colman rearrangement
7575:Bucherer carbazole synthesis
7570:Borsche–Drechsel cyclization
7550:Bernthsen acridine synthesis
7535:Bamberger triazine synthesis
7520:Algar–Flynn–Oyamada reaction
7231:Nazarov cyclization reaction
7096:De Kimpe aziridine synthesis
7051:Bucherer carbazole synthesis
7046:Borsche–Drechsel cyclization
6816:Nazarov cyclization reaction
6796:Meyer–Schuster rearrangement
6726:Gabriel–Colman rearrangement
6476:Wolffenstein–Böters reaction
6361:Reduction of nitro compounds
6211:Grundmann aldehyde synthesis
6016:Algar–Flynn–Oyamada reaction
5425:Olefin conversion technology
5420:Nozaki–Hiyama–Kishi reaction
5215:Gabriel–Colman rearrangement
5105:Claisen-Schmidt condensation
5050:Bouveault aldehyde synthesis
2371:
1032:to methylcyclohexenylketone.
585:
522:
7:
7635:Hantzsch pyridine synthesis
7414:Enone–alkene cycloadditions
7236:Nenitzescu indole synthesis
7156:Hantzsch pyridine synthesis
7121:Ferrario–Ackermann reaction
6771:Kowalski ester homologation
6736:Halogen dance rearrangement
6581:Benzilic acid rearrangement
6006:Akabori amino-acid reaction
5966:Von Braun amide degradation
5911:Barbier–Wieland degradation
5863:Nenitzescu indole synthesis
5843:Kharasch–Sosnovsky reaction
5734:Julia–Kocienski olefination
5638:Kowalski ester homologation
5335:Kowalski ester homologation
5310:Julia–Kocienski olefination
5065:Cadiot–Chodkiewicz coupling
4990:Aza-Baylis–Hillman reaction
4935:Acetoacetic ester synthesis
4646:Dynamic binding (chemistry)
4636:Conrotatory and disrotatory
4611:Charge remote fragmentation
2534:4-Methylcyclohexanemethanol
1289:10.1002/0471264180.or003.01
1214:
1003:1-β-phenylethylcyclohexanol
544:Friedel–Crafts dealkylation
192:reactions. Both proceed by
10:
7780:
7700:Robinson–Gabriel synthesis
7650:Kröhnke pyridine synthesis
7484:Retro-Diels–Alder reaction
7424:Imine Diels–Alder reaction
7211:Kröhnke pyridine synthesis
6826:Newman–Kwart rearrangement
6801:Mislow–Evans rearrangement
6711:Fischer–Hepp rearrangement
6656:Di-π-methane rearrangement
6436:Stephen aldehyde synthesis
6171:Eschweiler–Clarke reaction
5888:Williamson ether synthesis
5205:Fujiwara–Moritani reaction
5110:Combes quinoline synthesis
5075:Carbonyl olefin metathesis
4776:More O'Ferrall–Jencks plot
4701:Grunwald–Winstein equation
4671:Electron-withdrawing group
4606:Catalytic resonance theory
4372:Williamson ether synthesis
3671:2,4-Dichlorobenzyl alcohol
2602:2-(2-Methoxyethoxy)ethanol
2597:2-(2-Methoxyethoxy)ethanol
884:Arenes react with certain
214:Friedel-Crafts alkylation
7710:Urech hydantoin synthesis
7690:Pomeranz–Fritsch reaction
7615:Fischer oxazole synthesis
7512:
7349:1,3-Dipolar cycloaddition
7339:
7321:Urech hydantoin synthesis
7291:Reissert indole synthesis
7276:Pomeranz–Fritsch reaction
7206:Knorr quinoline synthesis
7136:Fischer oxazole synthesis
7066:Camps quinoline synthesis
6986:1,3-Dipolar cycloaddition
6974:
6886:Semipinacol rearrangement
6861:Ramberg–Bäcklund reaction
6846:Piancatelli rearrangement
6786:McFadyen–Stevens reaction
6541:Alpha-ketol rearrangement
6489:
6296:McFadyen–Stevens reaction
6241:Kiliani–Fischer synthesis
6161:Elbs persulfate oxidation
6086:Bouveault–Blanc reduction
6046:Baeyer–Villiger oxidation
5984:
5901:
5878:Schotten–Baumann reaction
5781:
5754:Ramberg–Bäcklund reaction
5661:
5633:Kiliani–Fischer synthesis
5613:
5475:Ramberg–Bäcklund reaction
5460:Pinacol coupling reaction
5455:Piancatelli rearrangement
5350:Liebeskind–Srogl coupling
5200:Fujimoto–Belleau reaction
4923:
4917:List of organic reactions
4781:Negative hyperconjugation
4526:
4468:
4409:
4391:Friedel-Crafts alkylation
4382:Nucleophilic substitution
4362:Nucleophilic substitution
4332:
4279:
4237:
4201:
4185:
4149:
4127:
4094:
4041:
4034:
3989:
3971:
3953:
3805:
3796:
3739:
3684:
3656:
3603:
3509:
3493:
3472:
3401:
3394:
3306:
3195:
3025:
2950:
2873:
2795:
2726:
2695:
2592:2-(2-Ethoxyethoxy)ethanol
2567:
2524:
2512:
2485:
2452:
2386:
2379:
737:
711:Organic Chemistry Portal
705:
677:
666:
659:
650:
639:
630:
621:
593:Friedel-Crafts acylation
592:
358:
337:friedel-crafts-alkylation
332:Organic Chemistry Portal
326:
298:
287:
280:
271:
260:
251:
242:
213:
158:
138:
110:
99:
90:
81:
72:
63:
34:
7685:Pictet–Spengler reaction
7600:Einhorn–Brunner reaction
7565:Boger pyridine synthesis
7459:Oxo-Diels–Alder reaction
7374:Aza-Diels–Alder reaction
7271:Pictet–Spengler reaction
7171:Hofmann–Löffler reaction
7161:Hegedus indole synthesis
7131:Fischer indole synthesis
7006:Bartoli indole synthesis
6961:Willgerodt rearrangement
6791:McLafferty rearrangement
6701:Ferrier carbocyclization
6516:2,3-Wittig rearrangement
6506:1,2-Wittig rearrangement
6346:Parikh–Doering oxidation
6336:Oxygen rebound mechanism
6001:Adkins–Peterson reaction
5893:Yamaguchi esterification
5833:Hegedus indole synthesis
5798:Bartoli indole synthesis
5669:Bamford–Stevens reaction
5585:Weinreb ketone synthesis
5545:Stork enamine alkylation
5320:Knoevenagel condensation
5190:Ferrier carbocyclization
5080:Castro–Stephens coupling
4706:Hammett acidity function
4696:Free-energy relationship
4641:Curtin–Hammett principle
4626:Conformational isomerism
3613:2,2-Dimethylpropan-1-ol
2669:-Diisopropylaminoethanol
2317:Perry C. Reeves (1977).
2128:Lee Irvin Smith (1930).
1929:10.1002/prac.18810230111
1699:Friedel-Crafts Acylation
1661:10.15227/orgsyn.013.0012
1595:10.1002/14356007.a19_313
1563:10.15227/orgsyn.032.0090
977:Friedel–Crafts arylation
945:Gattermann–Koch reaction
805:Gattermann-Koch reaction
716:friedel-crafts-acylation
166:Friedel–Crafts reactions
35:Friedel-Crafts reaction
7645:Knorr pyrrole synthesis
7580:Bucherer–Bergs reaction
7525:Allan–Robinson reaction
7504:Wagner-Jauregg reaction
7296:Ring-closing metathesis
7221:Larock indole synthesis
7201:Knorr pyrrole synthesis
7056:Bucherer–Bergs reaction
6921:Stieglitz rearrangement
6901:Skattebøl rearrangement
6871:Ring-closing metathesis
6731:Group transfer reaction
6696:Favorskii rearrangement
6636:Cornforth rearrangement
6566:Bamberger rearrangement
6471:Wolff–Kishner reduction
6291:Markó–Lam deoxygenation
6186:Fleming–Tamao oxidation
6181:Fischer–Tropsch process
5868:Oxymercuration reaction
5848:Knorr pyrrole synthesis
5674:Barton–Kellogg reaction
5580:Wagner-Jauregg reaction
5500:Ring-closing metathesis
5490:Reimer–Tiemann reaction
5480:Rauhut–Currier reaction
5395:Nef isocyanide reaction
5355:Malonic ester synthesis
5325:Knorr pyrrole synthesis
5260:High dilution principle
5195:Friedel–Crafts reaction
5130:Cross-coupling reaction
5055:Bucherer–Bergs reaction
5040:Blanc chloromethylation
5030:Blaise ketone synthesis
5005:Baylis–Hillman reaction
5000:Barton–Kellogg reaction
4975:Allan–Robinson reaction
4881:Woodward–Hoffmann rules
4616:Charge-transfer complex
4247:1,3-Difluoro-2-propanol
1589:. Weinheim: Wiley-VCH.
1174:Wolff-Kishner reduction
1083:
984:Blanc chloromethylation
938:Wolff-Kishner reduction
870:Wolff–Kishner reduction
839:, regenerating the AlCl
779:rearrangements, as the
580:2,6-di-tert-butylphenol
18:Friedel-Crafts reaction
7754:Substitution reactions
7610:Feist–Benary synthesis
7384:Bradsher cycloaddition
7354:4+4 Photocycloaddition
7311:Simmons–Smith reaction
7256:Paternò–Büchi reaction
7116:Feist–Benary synthesis
7106:Dieckmann condensation
6856:Pummerer rearrangement
6836:Oxy-Cope rearrangement
6811:Myers allene synthesis
6761:Jacobsen rearrangement
6676:Electrocyclic reaction
6651:Demjanov rearrangement
6606:Buchner ring expansion
6576:Beckmann rearrangement
6556:Aza-Cope rearrangement
6551:Arndt–Eistert reaction
6526:Alkyne zipper reaction
6446:Transfer hydrogenation
6421:Sharpless oxyamination
6396:Selenoxide elimination
6281:Lombardo methylenation
6206:Griesbaum coozonolysis
6116:Corey–Itsuno reduction
6091:Boyland–Sims oxidation
6031:Angeli–Rimini reaction
5679:Boord olefin synthesis
5623:Arndt–Eistert reaction
5615:Homologation reactions
5415:Nitro-Mannich reaction
5330:Kolbe–Schmitt reaction
5140:Cross-coupling partner
5060:Buchner ring expansion
4980:Arndt–Eistert reaction
4746:Kinetic isotope effect
4493:Rearrangement reaction
4252:2,2,2-Trifluoroethanol
2637:Aminoethylethanolamine
2587:2,2,2-Trifluoroethanol
2582:2,2,2-Trichloroethanol
2296:"β-methyanthraquinone"
2181:"trans-dienzoethylene"
1856:10.1002/anie.201210238
1830:Smith & March 2001
1763:Smith & March 2001
1751:Smith & March 2001
1420:Beilstein J. Org. Chem
1131:gives the fluorophore
959:Houben–Hoesch reaction
925:
567:
564:1,3-Diisopropylbenzene
537:
489:rearrangement reaction
203:
7469:Pauson–Khand reaction
7306:Sharpless epoxidation
7261:Pechmann condensation
7141:Friedländer synthesis
7091:Davis–Beirut reaction
6946:Wallach rearrangement
6916:Stevens rearrangement
6851:Pinacol rearrangement
6831:Overman rearrangement
6746:Hofmann rearrangement
6741:Hayashi rearrangement
6706:Ferrier rearrangement
6661:Dimroth rearrangement
6646:Curtius rearrangement
6641:Criegee rearrangement
6621:Claisen rearrangement
6611:Carroll rearrangement
6546:Amadori rearrangement
6536:Allylic rearrangement
6416:Sharpless epoxidation
6151:Dess–Martin oxidation
6076:Bohn–Schmidt reaction
5936:Hofmann rearrangement
5739:Kauffmann olefination
5662:Olefination reactions
5600:Wurtz–Fittig reaction
5435:Palladium–NHC complex
5315:Kauffmann olefination
5270:Homologation reaction
5120:Corey–House synthesis
5100:Claisen rearrangement
4896:Yukawa–Tsuno equation
4856:Swain–Lupton equation
4836:Spherical aromaticity
4771:Möbius–Hückel concept
4556:Aromatic ring current
4518:Substitution reaction
3676:3-Nitrobenzyl alcohol
2294:L. F. Fieser (1925).
2273:"p-bromoacetophenone"
2202:L. F. Fieser (1940).
991:Bogert–Cook synthesis
923:
761:aluminium trichloride
562:
535:
202:
7675:Paal–Knorr synthesis
7545:Barton–Zard reaction
7489:Staudinger synthesis
7439:Ketene cycloaddition
7409:Diels–Alder reaction
7389:Cheletropic reaction
7369:Alkyne trimerisation
7251:Paal–Knorr synthesis
7216:Kulinkovich reaction
7191:Jacobsen epoxidation
7111:Diels–Alder reaction
6906:Smiles rearrangement
6896:Sigmatropic reaction
6781:Lossen rearrangement
6631:Corey–Fuchs reaction
6596:Boekelheide reaction
6591:Bergmann degradation
6521:Achmatowicz reaction
6306:Methionine sulfoxide
6106:Clemmensen reduction
6066:Bergmann degradation
5996:Acyloin condensation
5961:Strecker degradation
5916:Bergmann degradation
5883:Ullmann condensation
5749:Peterson olefination
5724:Hydrazone iodination
5704:Elimination reaction
5605:Zincke–Suhl reaction
5525:Sonogashira coupling
5485:Reformatsky reaction
5445:Peterson olefination
5410:Nierenstein reaction
5340:Kulinkovich reaction
5155:Diels–Alder reaction
5115:Corey–Fuchs reaction
5095:Claisen condensation
4965:Alkyne trimerisation
4940:Acyloin condensation
4906:Σ-bishomoaromaticity
4866:Thorpe–Ingold effect
4478:Elimination reaction
4377:Elimination reaction
3079:1-Heptatetracontanol
3069:1-Pentatetracontanol
3064:1-Tetratetracontanol
2971:(melissyl / myricyl)
2938:(cluytyl / montanyl)
2647:Dimethylethanolamine
2017:Li, Jie Jack (2003)
1878:phosphorus pentoxide
1844:Angew. Chem. Int. Ed
1723:-Trimethylbenzoin".
1327:10.1039/cs9720100073
1170:Clemmensen reduction
1068:in an alkylation of
993:(1933) involves the
916:Scope and variations
874:Clemmensen reduction
7695:Prilezhaev reaction
7680:Pellizzari reaction
7359:(4+3) cycloaddition
7326:Van Leusen reaction
7301:Robinson annulation
7286:Pschorr cyclization
7281:Prilezhaev reaction
7011:Bergman cyclization
6966:Wolff rearrangement
6951:Weerman degradation
6841:Pericyclic reaction
6821:Neber rearrangement
6716:Fries rearrangement
6601:Brook rearrangement
6586:Bergman cyclization
6431:Staudinger reaction
6376:Rosenmund reduction
6366:Reductive amination
6331:Oppenauer oxidation
6121:Corey–Kim oxidation
6096:Cannizzaro reaction
5971:Weerman degradation
5946:Isosaccharinic acid
5858:Mukaiyama hydration
5714:Hofmann elimination
5699:Dehydrohalogenation
5684:Chugaev elimination
5505:Robinson annulation
5450:Pfitzinger reaction
5220:Gattermann reaction
5165:Wulff–Dötz reaction
5145:Dakin–West reaction
5070:Carbonyl allylation
5015:Bergman cyclization
4801:Kennedy J. P. Orton
4721:Hammond's postulate
4691:Flippin–Lodge angle
4661:Electromeric effect
4586:Beta-silicon effect
4571:Baker–Nathan effect
4401:Transesterification
3778:Palmitoleyl alcohol
3753:3-Methyl-3-pentanol
3696:Cetostearyl alcohol
3633:3-Methylbutan-2-ol
3628:3-Methylbutan-1-ol
3623:2-Methylbutan-2-ol
3618:2-Methylbutan-1-ol
3402:Monohydric alcohols
3331:3-Methyl-3-pentanol
3321:2-Methylheptan-2-ol
3316:2-Methyl-2-pentanol
3089:1-Nonatetracontanol
3084:1-Octatetracontanol
3074:1-Hexatetracontanol
3007:1-Heptatriacontanol
2997:1-Pentatriacontanol
2991:1-Tetratriacontanol
2677:-Methylethanolamine
2642:Diethylethanolamine
2554:Methylazoxymethanol
2471:Methanol poisoning
2179:R. E. Lutz (1940).
2059:10.1021/jo01301a007
1997:2007JChEd..84.1799M
1957:2007JChEd..84..692S
1896:10.1021/ja01297a514
1876:This reaction with
1775:Smith, Michael B.;
1738:10.1021/ja01313a015
1634:10.1021/jo01256a098
1517:Smith, Michael B.;
1127:in the presence of
966:Fries rearrangement
689:Strong lewis acid:
310:Strong lewis acid:
122:Strong lewis acid:
7749:Coupling reactions
7444:McCormack reaction
7394:Conia-ene reaction
7226:Madelung synthesis
7016:Biginelli reaction
6806:Mumm rearrangement
6691:Favorskii reaction
6626:Cope rearrangement
6616:Chan rearrangement
6381:Rubottom oxidation
6311:Miyaura borylation
6276:Lipid peroxidation
6271:Lindgren oxidation
6251:Kornblum oxidation
6246:Kolbe electrolysis
6191:Fukuyama reduction
6101:Carbonyl reduction
5951:Marker degradation
5813:Diazonium compound
5803:Boudouard reaction
5782:Carbon-heteroatom
5709:Grieco elimination
5495:Rieche formylation
5440:Passerini reaction
5370:Meerwein arylation
5290:Hydroxymethylation
5185:Favorskii reaction
5085:Chan rearrangement
5020:Biginelli reaction
4945:Aldol condensation
4791:2-Norbornyl cation
4766:Möbius aromaticity
4761:Markovnikov's rule
4656:Effective molarity
4601:Bürgi–Dunitz angle
4591:Bicycloaromaticity
4296:Carbonyl reduction
4172:Diethylpropanediol
3788:tert-Butyl alcohol
3494:Trihydric alcohols
3336:3-Methyloctan-3-ol
3326:2-Methylhexan-2-ol
3239:3-Methyl-2-butanol
3059:1-Tritetracontanol
3049:1-Hentetracontanol
3017:1-Nonatriacontanol
3012:1-Octatriacontanol
3002:1-Hexatriacontanol
2850:(cetyl / palmityl)
2705:2-Methyl-1-butanol
2544:Cyclohexylmethanol
2403:2-Methyl-1-butanol
2005:10.1021/ed084p1799
1209:aluminium chloride
1166:succinic anhydride
1109:phthalic anhydride
1062:aluminium chloride
952:Gatterman reaction
926:
827:Reaction mechanism
817:aluminium chloride
568:
538:
504:methallyl chloride
442:aluminium chloride
412:With alkyl halides
204:
7736:
7735:
7732:
7731:
7728:
7727:
7720:Wohl–Aue reaction
7364:6+4 Cycloaddition
7181:Iodolactonization
6501:1,2-rearrangement
6466:Wohl–Aue reaction
6386:Sabatier reaction
6351:Pinnick oxidation
6316:Mozingo reduction
6261:Leuckart reaction
6216:Haloform reaction
6131:Criegee oxidation
6111:Collins oxidation
6061:Benkeser reaction
6056:Bechamp reduction
6026:Andrussow process
6011:Alcohol oxidation
5921:Edman degradation
5828:Haloform reaction
5777:
5776:
5764:Takai olefination
5729:Julia olefination
5555:Takai olefination
5430:Olefin metathesis
5305:Julia olefination
5230:Grignard reaction
5210:Fukuyama coupling
5125:Coupling reaction
5090:Chan–Lam coupling
4960:Alkyne metathesis
4955:Alkane metathesis
4811:Phosphaethynolate
4716:George S. Hammond
4676:Electronic effect
4631:Conjugated system
4513:Stereospecificity
4508:Stereoselectivity
4473:Addition reaction
4462:organic reactions
4427:
4426:
4350:Alcohol oxidation
4271:Trifluoromethanol
4145:
4144:
4030:
4029:
3783:tert-Amyl alcohol
3763:Linolenyl alcohol
3658:Aromatic alcohols
3599:
3598:
3473:Dihydric alcohols
3386:Triphenylmethanol
3341:Diacetone alcohol
3289:Pinacolyl alcohol
3214:2-Deoxyerythritol
3187:Veratrole alcohol
3167:Propargyl alcohol
3157:Phenethyl alcohol
3147:Nicotinyl alcohol
3142:Neopentyl alcohol
3097:
3096:
3054:1-Dotetracontanol
2986:1-Tritriacontanol
2975:1-Hentriacontanol
2627:2-Mercaptoethanol
2559:Trifluoromethanol
2508:
2507:
2495:Isopropyl alcohol
2423:Phenethyl alcohol
2388:Alcohols found in
2327:Organic Syntheses
2304:Organic Syntheses
2281:Organic Syntheses
2258:Organic Syntheses
2235:Organic Syntheses
2212:Organic Syntheses
2189:Organic Syntheses
2161:Organic Syntheses
2138:Organic Syntheses
2115:Organic Syntheses
2107:"Diphenylacetone"
2095:Organic Syntheses
2085:978-1-4390-4914-3
2053:(13): 2581–2585.
1965:10.1021/ed084p692
1850:(38): 9900–9930.
1792:978-0-471-72091-1
1732:(10): 1803–1804.
1726:J. Am. Chem. Soc.
1649:Organic Syntheses
1550:Organic Syntheses
1534:978-0-471-72091-1
1400:978-0-08-052349-1
1379:Compr. Org. Synth
1363:978-0-08-052349-1
1342:Compr. Org. Synth
1227:Hydrodealkylation
1158:Haworth synthesis
1152:Haworth synthesis
880:Hydroxyalkylation
813:hydrogen chloride
741:
740:
673:
672:
616:Coupling reaction
572:protecting groups
502:from benzene and
484:) will undergo a
408:as the catalyst.
362:
361:
294:
293:
237:Coupling reaction
162:
161:
106:
105:
58:Coupling reaction
16:(Redirected from
7771:
7715:Wenker synthesis
7705:Stollé synthesis
7560:Bobbitt reaction
7530:Auwers synthesis
7474:Povarov reaction
7399:Cyclopropanation
7337:
7336:
7331:Wenker synthesis
7086:Darzens reaction
7036:Bobbitt reaction
6881:Schmidt reaction
6686:Enyne metathesis
6461:Whiting reaction
6456:Wharton reaction
6401:Shapiro reaction
6391:Sarett oxidation
6356:Prévost reaction
6166:Emde degradation
5976:Wohl degradation
5956:Ruff degradation
5926:Emde degradation
5823:Grignard reagent
5759:Shapiro reaction
5744:McMurry reaction
5611:
5610:
5575:Ullmann reaction
5540:Stollé synthesis
5530:Stetter reaction
5520:Shapiro reaction
5510:Sakurai reaction
5405:Negishi coupling
5385:Minisci reaction
5380:Michael reaction
5365:McMurry reaction
5360:Mannich reaction
5240:Hammick reaction
5235:Grignard reagent
5175:Enyne metathesis
5160:Doebner reaction
5150:Darzens reaction
4995:Barbier reaction
4985:Auwers synthesis
4912:
4911:
4886:Woodward's rules
4851:Superaromaticity
4841:Spiroaromaticity
4741:Inductive effect
4736:Hyperconjugation
4711:Hammett equation
4651:Edwards equation
4503:Regioselectivity
4454:
4447:
4440:
4431:
4430:
4417:
4416:
4039:
4038:
4035:Terpene alcohols
3820:Methylene glycol
3803:
3802:
3768:Linoleyl alcohol
3711:Myristyl alcohol
3538:Propylene glycol
3485:Propylene glycol
3399:
3398:
3381:Triphenylethanol
3294:Pirkle's alcohol
3279:Diphenylmethanol
3182:Vanillyl alcohol
3152:Perillyl alcohol
3132:Furfuryl alcohol
3122:Cinnamyl alcohol
2980:1-Dotriacontanol
2632:2-Methoxyethanol
2522:
2521:
2390:alcoholic drinks
2384:
2383:
2366:
2359:
2352:
2343:
2342:
2335:
2323:
2312:
2300:
2289:
2277:
2266:
2254:
2243:
2231:
2220:
2208:
2197:
2185:
2169:
2157:
2146:
2134:
2123:
2111:
2087:
2077:
2071:
2070:
2038:
2032:
2015:
2009:
2008:
1975:
1969:
1968:
1939:
1933:
1932:
1906:
1900:
1899:
1890:(6): 1062–1063.
1874:
1868:
1867:
1839:
1833:
1827:
1821:
1820:
1802:
1796:
1795:
1772:
1766:
1760:
1754:
1748:
1742:
1741:
1722:
1718:
1714:
1708:
1702:
1696:
1690:
1689:
1687:
1685:
1671:
1665:
1664:
1644:
1638:
1637:
1628:(4): 1160–1161.
1615:
1609:
1608:
1582:
1576:
1573:
1567:
1565:
1544:
1538:
1537:
1514:
1505:
1504:
1494:
1486:
1460:
1454:
1453:
1443:
1432:10.3762/bjoc.6.6
1411:
1405:
1404:
1374:
1368:
1367:
1337:
1331:
1330:
1309:
1303:
1302:
1272:
1266:
1247:
1184:
1164:is reacted with
1147:
1119:
1016:
904:
864:
844:quantity of AlCl
821:cuprous chloride
791:
733:
718:
654:
644:Acylating agents
628:
627:
590:
589:
518:
500:neophyl chloride
471:
457:steric hindrance
396:
389:
354:
339:
275:
265:Alkylating Agent
249:
248:
211:
210:
154:
101:Coupling Product
94:
70:
69:
32:
31:
21:
7779:
7778:
7774:
7773:
7772:
7770:
7769:
7768:
7739:
7738:
7737:
7724:
7625:Gewald reaction
7508:
7335:
7316:Skraup reaction
7151:Graham reaction
7146:Gewald reaction
6977:
6970:
6492:
6485:
6441:Swern oxidation
6426:Stahl oxidation
6371:Riley oxidation
6326:Omega oxidation
6286:Luche reduction
6236:Jones oxidation
6201:Glycol cleavage
6196:Ganem oxidation
6141:Davis oxidation
6136:Dakin oxidation
6071:Birch reduction
6021:Amide reduction
5987:
5980:
5941:Hooker reaction
5903:
5897:
5785:
5783:
5773:
5769:Wittig reaction
5657:
5653:Wittig reaction
5628:Hooker reaction
5609:
5590:Wittig reaction
5565:Thorpe reaction
5550:Suzuki reaction
5535:Stille reaction
5470:Quelet reaction
5345:Kumada coupling
5295:Ivanov reaction
5285:Hydrovinylation
5265:Hiyama coupling
5225:Glaser coupling
5035:Blaise reaction
5025:Bingel reaction
5010:Benary reaction
4927:
4925:
4919:
4910:
4806:Passive binding
4726:Homoaromaticity
4576:Baldwin's rules
4551:Antiaromaticity
4546:Anomeric effect
4522:
4464:
4458:
4428:
4423:
4405:
4355:Glycol cleavage
4328:
4324:Ziegler process
4275:
4257:2-Fluoroethanol
4233:
4197:
4181:
4177:Ethylene glycol
4162:1,5-Pentanediol
4141:
4129:
4123:
4096:
4090:
4043:
4026:
3990:Glycylglycitols
3985:
3973:
3967:
3955:
3949:
3945:
3935:
3925:
3915:
3905:
3895:
3885:
3875:
3865:
3855:
3845:
3835:
3830:Ethylene glycol
3825:
3813:
3809:
3792:
3743:
3741:
3735:
3686:
3680:
3652:
3595:
3591:
3581:
3567:
3557:
3543:
3529:
3524:Ethylene glycol
3519:Pentaerythritol
3505:
3489:
3480:Ethylene glycol
3468:
3464:
3454:
3444:
3434:
3424:
3414:
3395:Hydric alcohols
3390:
3308:
3302:
3204:1-Phenylethanol
3197:
3191:
3172:Salicyl alcohol
3137:Isoamyl alcohol
3093:
3044:1-Tetracontanol
3037:
3033:
3029:
3027:
3021:
2962:
2958:
2954:
2952:
2946:
2885:
2881:
2877:
2875:
2869:
2807:
2803:
2799:
2797:
2791:
2738:
2734:
2730:
2728:
2722:
2691:
2687:Tribromoethanol
2622:2-Fluoroethanol
2617:2-Ethoxyethanol
2612:2-Chloroethanol
2607:2-Butoxyethanol
2563:
2549:Methoxymethanol
2515:
2504:
2481:
2454:Medical alcohol
2448:
2413:Isoamyl alcohol
2389:
2375:
2370:
2321:
2298:
2275:
2252:
2229:
2227:"Desoxybenzoin"
2206:
2183:
2155:
2132:
2109:
2098:
2090:
2078:
2074:
2039:
2035:
2016:
2012:
1976:
1972:
1940:
1936:
1916:J. Prakt. Chem.
1907:
1903:
1875:
1871:
1840:
1836:
1828:
1824:
1817:
1809:. p. 725.
1803:
1799:
1793:
1773:
1769:
1761:
1757:
1753:, p. 1835.
1749:
1745:
1720:
1716:
1712:
1709:
1705:
1697:
1693:
1683:
1681:
1675:"Desoxybenzoin"
1673:
1672:
1668:
1645:
1641:
1616:
1612:
1605:
1583:
1579:
1574:
1570:
1545:
1541:
1535:
1515:
1508:
1488:
1487:
1475:
1461:
1457:
1412:
1408:
1401:
1375:
1371:
1364:
1338:
1334:
1315:Chem. Soc. Rev.
1310:
1306:
1299:
1273:
1269:
1248:
1244:
1240:
1232:Transalkylation
1217:
1201:
1193:
1154:
1101:thymolphthalein
1086:
1064:is replaced by
1058:green chemistry
1051:Nencki reaction
1035:In the related
1030:acetyl chloride
973:Scholl reaction
918:
882:
851:
847:
842:
838:
829:
809:carbon monoxide
766:
753:Acid anhydrides
729:
714:
701:
699:
690:
668:
652:
641:
604:
602:Charles Friedel
588:
554:transalkylation
546:
525:
483:
414:
404:derived from a
367:
350:
335:
322:
320:
311:
289:
273:
262:
225:
223:Charles Friedel
209:
174:Charles Friedel
150:
134:
132:
123:
92:
83:
46:
44:Charles Friedel
28:
23:
22:
15:
12:
11:
5:
7777:
7767:
7766:
7764:Name reactions
7761:
7756:
7751:
7734:
7733:
7730:
7729:
7726:
7725:
7723:
7722:
7717:
7712:
7707:
7702:
7697:
7692:
7687:
7682:
7677:
7672:
7667:
7662:
7657:
7652:
7647:
7642:
7637:
7632:
7630:Hantzsch ester
7627:
7622:
7617:
7612:
7607:
7602:
7597:
7592:
7587:
7582:
7577:
7572:
7567:
7562:
7557:
7552:
7547:
7542:
7540:Banert cascade
7537:
7532:
7527:
7522:
7516:
7514:
7510:
7509:
7507:
7506:
7501:
7496:
7491:
7486:
7481:
7479:Prato reaction
7476:
7471:
7466:
7461:
7456:
7451:
7446:
7441:
7436:
7431:
7426:
7421:
7416:
7411:
7406:
7401:
7396:
7391:
7386:
7381:
7376:
7371:
7366:
7361:
7356:
7351:
7345:
7343:
7334:
7333:
7328:
7323:
7318:
7313:
7308:
7303:
7298:
7293:
7288:
7283:
7278:
7273:
7268:
7263:
7258:
7253:
7248:
7243:
7238:
7233:
7228:
7223:
7218:
7213:
7208:
7203:
7198:
7193:
7188:
7183:
7178:
7173:
7168:
7163:
7158:
7153:
7148:
7143:
7138:
7133:
7128:
7123:
7118:
7113:
7108:
7103:
7098:
7093:
7088:
7083:
7078:
7073:
7068:
7063:
7058:
7053:
7048:
7043:
7038:
7033:
7028:
7023:
7018:
7013:
7008:
7003:
6998:
6993:
6988:
6982:
6980:
6972:
6971:
6969:
6968:
6963:
6958:
6953:
6948:
6943:
6938:
6933:
6928:
6923:
6918:
6913:
6908:
6903:
6898:
6893:
6888:
6883:
6878:
6873:
6868:
6863:
6858:
6853:
6848:
6843:
6838:
6833:
6828:
6823:
6818:
6813:
6808:
6803:
6798:
6793:
6788:
6783:
6778:
6773:
6768:
6763:
6758:
6753:
6748:
6743:
6738:
6733:
6728:
6723:
6718:
6713:
6708:
6703:
6698:
6693:
6688:
6683:
6678:
6673:
6668:
6663:
6658:
6653:
6648:
6643:
6638:
6633:
6628:
6623:
6618:
6613:
6608:
6603:
6598:
6593:
6588:
6583:
6578:
6573:
6571:Banert cascade
6568:
6563:
6558:
6553:
6548:
6543:
6538:
6533:
6528:
6523:
6518:
6513:
6508:
6503:
6497:
6495:
6491:Rearrangement
6487:
6486:
6484:
6483:
6481:Zinin reaction
6478:
6473:
6468:
6463:
6458:
6453:
6451:Wacker process
6448:
6443:
6438:
6433:
6428:
6423:
6418:
6413:
6408:
6403:
6398:
6393:
6388:
6383:
6378:
6373:
6368:
6363:
6358:
6353:
6348:
6343:
6338:
6333:
6328:
6323:
6318:
6313:
6308:
6303:
6298:
6293:
6288:
6283:
6278:
6273:
6268:
6263:
6258:
6253:
6248:
6243:
6238:
6233:
6228:
6226:Hydrogenolysis
6223:
6218:
6213:
6208:
6203:
6198:
6193:
6188:
6183:
6178:
6176:Étard reaction
6173:
6168:
6163:
6158:
6153:
6148:
6143:
6138:
6133:
6128:
6123:
6118:
6113:
6108:
6103:
6098:
6093:
6088:
6083:
6081:Bosch reaction
6078:
6073:
6068:
6063:
6058:
6053:
6048:
6043:
6038:
6033:
6028:
6023:
6018:
6013:
6008:
6003:
5998:
5992:
5990:
5986:Organic redox
5982:
5981:
5979:
5978:
5973:
5968:
5963:
5958:
5953:
5948:
5943:
5938:
5933:
5928:
5923:
5918:
5913:
5907:
5905:
5899:
5898:
5896:
5895:
5890:
5885:
5880:
5875:
5870:
5865:
5860:
5855:
5850:
5845:
5840:
5835:
5830:
5825:
5820:
5818:Esterification
5815:
5810:
5805:
5800:
5795:
5789:
5787:
5779:
5778:
5775:
5774:
5772:
5771:
5766:
5761:
5756:
5751:
5746:
5741:
5736:
5731:
5726:
5721:
5716:
5711:
5706:
5701:
5696:
5691:
5686:
5681:
5676:
5671:
5665:
5663:
5659:
5658:
5656:
5655:
5650:
5645:
5640:
5635:
5630:
5625:
5619:
5617:
5608:
5607:
5602:
5597:
5595:Wurtz reaction
5592:
5587:
5582:
5577:
5572:
5567:
5562:
5557:
5552:
5547:
5542:
5537:
5532:
5527:
5522:
5517:
5512:
5507:
5502:
5497:
5492:
5487:
5482:
5477:
5472:
5467:
5465:Prins reaction
5462:
5457:
5452:
5447:
5442:
5437:
5432:
5427:
5422:
5417:
5412:
5407:
5402:
5397:
5392:
5387:
5382:
5377:
5372:
5367:
5362:
5357:
5352:
5347:
5342:
5337:
5332:
5327:
5322:
5317:
5312:
5307:
5302:
5297:
5292:
5287:
5282:
5280:Hydrocyanation
5277:
5272:
5267:
5262:
5257:
5252:
5250:Henry reaction
5247:
5242:
5237:
5232:
5227:
5222:
5217:
5212:
5207:
5202:
5197:
5192:
5187:
5182:
5177:
5172:
5167:
5162:
5157:
5152:
5147:
5142:
5137:
5132:
5127:
5122:
5117:
5112:
5107:
5102:
5097:
5092:
5087:
5082:
5077:
5072:
5067:
5062:
5057:
5052:
5047:
5042:
5037:
5032:
5027:
5022:
5017:
5012:
5007:
5002:
4997:
4992:
4987:
4982:
4977:
4972:
4967:
4962:
4957:
4952:
4950:Aldol reaction
4947:
4942:
4937:
4931:
4929:
4924:Carbon-carbon
4921:
4920:
4909:
4908:
4903:
4901:Zaitsev's rule
4898:
4893:
4888:
4883:
4878:
4873:
4868:
4863:
4858:
4853:
4848:
4846:Steric effects
4843:
4838:
4833:
4828:
4823:
4818:
4813:
4808:
4803:
4798:
4793:
4788:
4783:
4778:
4773:
4768:
4763:
4758:
4753:
4748:
4743:
4738:
4733:
4728:
4723:
4718:
4713:
4708:
4703:
4698:
4693:
4688:
4683:
4678:
4673:
4668:
4663:
4658:
4653:
4648:
4643:
4638:
4633:
4628:
4623:
4618:
4613:
4608:
4603:
4598:
4593:
4588:
4583:
4578:
4573:
4568:
4563:
4558:
4553:
4548:
4543:
4538:
4533:
4527:
4524:
4523:
4521:
4520:
4515:
4510:
4505:
4500:
4498:Redox reaction
4495:
4490:
4485:
4483:Polymerization
4480:
4475:
4469:
4466:
4465:
4457:
4456:
4449:
4442:
4434:
4425:
4424:
4422:
4421:
4410:
4407:
4406:
4404:
4403:
4398:
4393:
4388:
4386:carbonyl group
4379:
4374:
4369:
4364:
4359:
4358:
4357:
4347:
4342:
4336:
4334:
4330:
4329:
4327:
4326:
4321:
4312:
4303:
4301:Ether cleavage
4298:
4293:
4283:
4281:
4277:
4276:
4274:
4273:
4268:
4266:-butyl alcohol
4259:
4254:
4249:
4243:
4241:
4239:Fluoroalcohols
4235:
4234:
4232:
4231:
4226:
4221:
4216:
4211:
4205:
4203:
4199:
4198:
4196:
4195:
4189:
4187:
4183:
4182:
4180:
4179:
4174:
4169:
4164:
4159:
4157:1,4-Butanediol
4153:
4151:
4147:
4146:
4143:
4142:
4140:
4139:
4133:
4131:
4125:
4124:
4122:
4121:
4116:
4111:
4106:
4100:
4098:
4092:
4091:
4089:
4088:
4083:
4078:
4073:
4068:
4063:
4058:
4053:
4047:
4045:
4036:
4032:
4031:
4028:
4027:
4025:
4024:
4019:
4017:Maltotetraitol
4014:
4009:
4004:
3999:
3993:
3991:
3987:
3986:
3984:
3983:
3977:
3975:
3969:
3968:
3966:
3965:
3959:
3957:
3951:
3950:
3948:
3947:
3943:
3937:
3933:
3927:
3923:
3917:
3913:
3907:
3903:
3897:
3893:
3887:
3883:
3877:
3873:
3867:
3863:
3857:
3853:
3847:
3843:
3837:
3833:
3827:
3823:
3816:
3814:
3811:
3807:
3800:
3798:Sugar alcohols
3794:
3793:
3791:
3790:
3785:
3780:
3775:
3770:
3765:
3760:
3758:Erucyl alcohol
3755:
3749:
3747:
3745:fatty alcohols
3737:
3736:
3734:
3733:
3728:
3723:
3718:
3713:
3708:
3706:Lauryl alcohol
3703:
3698:
3692:
3690:
3688:fatty alcohols
3682:
3681:
3679:
3678:
3673:
3668:
3666:Benzyl alcohol
3662:
3660:
3654:
3653:
3651:
3650:
3645:
3640:
3635:
3630:
3625:
3620:
3615:
3609:
3607:
3601:
3600:
3597:
3596:
3594:
3593:
3589:
3583:
3579:
3569:
3565:
3559:
3555:
3545:
3541:
3531:
3527:
3521:
3515:
3513:
3507:
3506:
3504:
3503:
3497:
3495:
3491:
3490:
3488:
3487:
3482:
3476:
3474:
3470:
3469:
3467:
3466:
3462:
3456:
3452:
3446:
3442:
3436:
3432:
3426:
3422:
3416:
3412:
3405:
3403:
3396:
3392:
3391:
3389:
3388:
3383:
3378:
3376:-Butyl alcohol
3370:
3362:
3360:-butyl alcohol
3353:
3351:Methylpentynol
3348:
3343:
3338:
3333:
3328:
3323:
3318:
3312:
3310:
3304:
3303:
3301:
3296:
3291:
3286:
3281:
3276:
3271:
3266:
3261:
3256:
3251:
3246:
3241:
3236:
3231:
3226:
3221:
3216:
3211:
3206:
3201:
3199:
3193:
3192:
3190:
3189:
3184:
3179:
3174:
3169:
3164:
3159:
3154:
3149:
3144:
3139:
3134:
3129:
3127:Crotyl alcohol
3124:
3119:
3117:Benzyl alcohol
3114:
3112:Anisyl alcohol
3109:
3104:
3102:2-Ethylhexanol
3098:
3095:
3094:
3092:
3091:
3086:
3081:
3076:
3071:
3066:
3061:
3056:
3051:
3046:
3040:
3038:
3035:
3031:
3026:Straight-chain
3023:
3022:
3020:
3019:
3014:
3009:
3004:
2999:
2994:
2988:
2983:
2977:
2972:
2969:1-Triacontanol
2965:
2963:
2960:
2956:
2951:Straight-chain
2948:
2947:
2945:
2944:
2939:
2933:
2931:1-Heptacosanol
2928:
2922:
2920:1-Pentacosanol
2917:
2914:1-Tetracosanol
2911:
2906:
2900:
2898:1-Heneicosanol
2895:
2888:
2886:
2883:
2879:
2874:Straight-chain
2871:
2870:
2868:
2867:
2862:
2856:
2854:1-Heptadecanol
2851:
2845:
2843:1-Pentadecanol
2840:
2837:1-Tetradecanol
2834:
2829:
2823:
2817:
2810:
2808:
2805:
2801:
2796:Straight-chain
2793:
2792:
2790:
2789:
2783:
2777:
2772:
2767:
2762:
2757:
2752:
2747:
2741:
2739:
2736:
2732:
2727:Straight-chain
2724:
2723:
2721:
2720:
2712:
2707:
2701:
2699:
2693:
2692:
2690:
2689:
2684:
2682:Phenoxyethanol
2679:
2671:
2659:
2654:
2649:
2644:
2639:
2634:
2629:
2624:
2619:
2614:
2609:
2604:
2599:
2594:
2589:
2584:
2579:
2577:1-Aminoethanol
2573:
2571:
2565:
2564:
2562:
2561:
2556:
2551:
2546:
2541:
2536:
2530:
2528:
2519:
2510:
2509:
2506:
2505:
2503:
2502:
2497:
2491:
2489:
2487:Toxic alcohols
2483:
2482:
2480:
2479:
2478:
2477:
2469:
2467:Methylpentynol
2464:
2458:
2456:
2450:
2449:
2447:
2446:
2441:
2439:-Butyl alcohol
2433:
2425:
2420:
2415:
2410:
2405:
2400:
2394:
2392:
2381:
2380:By consumption
2377:
2376:
2369:
2368:
2361:
2354:
2346:
2340:
2339:
2338:
2337:
2314:
2291:
2268:
2245:
2222:
2199:
2173:
2172:
2171:
2153:"Benzophenone"
2148:
2125:
2097:
2091:
2089:
2088:
2072:
2033:
2010:
1985:J. Chem. Educ.
1970:
1945:J. Chem. Educ.
1934:
1901:
1869:
1834:
1832:, p. 732.
1822:
1815:
1797:
1791:
1767:
1765:, p. 745.
1755:
1743:
1703:
1691:
1666:
1639:
1610:
1603:
1577:
1568:
1539:
1533:
1506:
1473:
1455:
1406:
1399:
1369:
1362:
1332:
1304:
1297:
1267:
1241:
1239:
1236:
1235:
1234:
1229:
1224:
1221:Friedel family
1216:
1213:
1200:
1197:
1191:
1186:
1185:
1153:
1150:
1149:
1148:
1121:
1120:
1090:triarylmethane
1085:
1082:
1081:
1080:
1054:
1047:
1033:
1010:
1009:
987:
980:
969:
962:
955:
948:
941:
917:
914:
906:
905:
896:with benzene:
892:derivative of
881:
878:
866:
865:
849:
845:
840:
836:
828:
825:
793:
792:
764:
749:acyl chlorides
739:
738:
735:
734:
727:
720:
719:
712:
708:
707:
703:
702:
697:
688:
686:
680:
679:
675:
674:
671:
670:
664:
663:
657:
656:
648:
647:
637:
636:
624:
623:
619:
618:
613:
612:Reaction type
609:
608:
599:
595:
594:
587:
584:
545:
542:
541:
540:
524:
521:
520:
519:
481:
473:
472:
413:
410:
398:
397:
390:
379:cumene process
366:
363:
360:
359:
356:
355:
348:
341:
340:
333:
329:
328:
324:
323:
318:
309:
307:
301:
300:
296:
295:
292:
291:
285:
284:
278:
277:
269:
268:
258:
257:
245:
244:
240:
239:
234:
233:Reaction type
230:
229:
220:
216:
215:
208:
205:
188:reactions and
160:
159:
156:
155:
148:
141:
140:
136:
135:
130:
121:
119:
113:
112:
108:
107:
104:
103:
97:
96:
88:
87:
79:
78:
66:
65:
61:
60:
55:
54:Reaction type
51:
50:
41:
37:
36:
26:
9:
6:
4:
3:
2:
7776:
7765:
7762:
7760:
7757:
7755:
7752:
7750:
7747:
7746:
7744:
7721:
7718:
7716:
7713:
7711:
7708:
7706:
7703:
7701:
7698:
7696:
7693:
7691:
7688:
7686:
7683:
7681:
7678:
7676:
7673:
7671:
7668:
7666:
7663:
7661:
7658:
7656:
7653:
7651:
7648:
7646:
7643:
7641:
7640:Herz reaction
7638:
7636:
7633:
7631:
7628:
7626:
7623:
7621:
7618:
7616:
7613:
7611:
7608:
7606:
7603:
7601:
7598:
7596:
7593:
7591:
7588:
7586:
7583:
7581:
7578:
7576:
7573:
7571:
7568:
7566:
7563:
7561:
7558:
7556:
7553:
7551:
7548:
7546:
7543:
7541:
7538:
7536:
7533:
7531:
7528:
7526:
7523:
7521:
7518:
7517:
7515:
7511:
7505:
7502:
7500:
7497:
7495:
7492:
7490:
7487:
7485:
7482:
7480:
7477:
7475:
7472:
7470:
7467:
7465:
7462:
7460:
7457:
7455:
7452:
7450:
7447:
7445:
7442:
7440:
7437:
7435:
7432:
7430:
7427:
7425:
7422:
7420:
7417:
7415:
7412:
7410:
7407:
7405:
7402:
7400:
7397:
7395:
7392:
7390:
7387:
7385:
7382:
7380:
7377:
7375:
7372:
7370:
7367:
7365:
7362:
7360:
7357:
7355:
7352:
7350:
7347:
7346:
7344:
7342:
7341:Cycloaddition
7338:
7332:
7329:
7327:
7324:
7322:
7319:
7317:
7314:
7312:
7309:
7307:
7304:
7302:
7299:
7297:
7294:
7292:
7289:
7287:
7284:
7282:
7279:
7277:
7274:
7272:
7269:
7267:
7264:
7262:
7259:
7257:
7254:
7252:
7249:
7247:
7244:
7242:
7239:
7237:
7234:
7232:
7229:
7227:
7224:
7222:
7219:
7217:
7214:
7212:
7209:
7207:
7204:
7202:
7199:
7197:
7194:
7192:
7189:
7187:
7186:Isay reaction
7184:
7182:
7179:
7177:
7174:
7172:
7169:
7167:
7164:
7162:
7159:
7157:
7154:
7152:
7149:
7147:
7144:
7142:
7139:
7137:
7134:
7132:
7129:
7127:
7124:
7122:
7119:
7117:
7114:
7112:
7109:
7107:
7104:
7102:
7099:
7097:
7094:
7092:
7089:
7087:
7084:
7082:
7081:Cycloaddition
7079:
7077:
7074:
7072:
7069:
7067:
7064:
7062:
7059:
7057:
7054:
7052:
7049:
7047:
7044:
7042:
7039:
7037:
7034:
7032:
7029:
7027:
7024:
7022:
7019:
7017:
7014:
7012:
7009:
7007:
7004:
7002:
6999:
6997:
6994:
6992:
6989:
6987:
6984:
6983:
6981:
6979:
6976:Ring forming
6973:
6967:
6964:
6962:
6959:
6957:
6954:
6952:
6949:
6947:
6944:
6942:
6939:
6937:
6934:
6932:
6929:
6927:
6924:
6922:
6919:
6917:
6914:
6912:
6909:
6907:
6904:
6902:
6899:
6897:
6894:
6892:
6889:
6887:
6884:
6882:
6879:
6877:
6876:Rupe reaction
6874:
6872:
6869:
6867:
6864:
6862:
6859:
6857:
6854:
6852:
6849:
6847:
6844:
6842:
6839:
6837:
6834:
6832:
6829:
6827:
6824:
6822:
6819:
6817:
6814:
6812:
6809:
6807:
6804:
6802:
6799:
6797:
6794:
6792:
6789:
6787:
6784:
6782:
6779:
6777:
6774:
6772:
6769:
6767:
6764:
6762:
6759:
6757:
6754:
6752:
6749:
6747:
6744:
6742:
6739:
6737:
6734:
6732:
6729:
6727:
6724:
6722:
6719:
6717:
6714:
6712:
6709:
6707:
6704:
6702:
6699:
6697:
6694:
6692:
6689:
6687:
6684:
6682:
6679:
6677:
6674:
6672:
6669:
6667:
6664:
6662:
6659:
6657:
6654:
6652:
6649:
6647:
6644:
6642:
6639:
6637:
6634:
6632:
6629:
6627:
6624:
6622:
6619:
6617:
6614:
6612:
6609:
6607:
6604:
6602:
6599:
6597:
6594:
6592:
6589:
6587:
6584:
6582:
6579:
6577:
6574:
6572:
6569:
6567:
6564:
6562:
6559:
6557:
6554:
6552:
6549:
6547:
6544:
6542:
6539:
6537:
6534:
6532:
6529:
6527:
6524:
6522:
6519:
6517:
6514:
6512:
6509:
6507:
6504:
6502:
6499:
6498:
6496:
6494:
6488:
6482:
6479:
6477:
6474:
6472:
6469:
6467:
6464:
6462:
6459:
6457:
6454:
6452:
6449:
6447:
6444:
6442:
6439:
6437:
6434:
6432:
6429:
6427:
6424:
6422:
6419:
6417:
6414:
6412:
6409:
6407:
6404:
6402:
6399:
6397:
6394:
6392:
6389:
6387:
6384:
6382:
6379:
6377:
6374:
6372:
6369:
6367:
6364:
6362:
6359:
6357:
6354:
6352:
6349:
6347:
6344:
6342:
6339:
6337:
6334:
6332:
6329:
6327:
6324:
6322:
6319:
6317:
6314:
6312:
6309:
6307:
6304:
6302:
6299:
6297:
6294:
6292:
6289:
6287:
6284:
6282:
6279:
6277:
6274:
6272:
6269:
6267:
6266:Ley oxidation
6264:
6262:
6259:
6257:
6254:
6252:
6249:
6247:
6244:
6242:
6239:
6237:
6234:
6232:
6231:Hydroxylation
6229:
6227:
6224:
6222:
6221:Hydrogenation
6219:
6217:
6214:
6212:
6209:
6207:
6204:
6202:
6199:
6197:
6194:
6192:
6189:
6187:
6184:
6182:
6179:
6177:
6174:
6172:
6169:
6167:
6164:
6162:
6159:
6157:
6156:DNA oxidation
6154:
6152:
6149:
6147:
6146:Deoxygenation
6144:
6142:
6139:
6137:
6134:
6132:
6129:
6127:
6124:
6122:
6119:
6117:
6114:
6112:
6109:
6107:
6104:
6102:
6099:
6097:
6094:
6092:
6089:
6087:
6084:
6082:
6079:
6077:
6074:
6072:
6069:
6067:
6064:
6062:
6059:
6057:
6054:
6052:
6049:
6047:
6044:
6042:
6039:
6037:
6036:Aromatization
6034:
6032:
6029:
6027:
6024:
6022:
6019:
6017:
6014:
6012:
6009:
6007:
6004:
6002:
5999:
5997:
5994:
5993:
5991:
5989:
5983:
5977:
5974:
5972:
5969:
5967:
5964:
5962:
5959:
5957:
5954:
5952:
5949:
5947:
5944:
5942:
5939:
5937:
5934:
5932:
5929:
5927:
5924:
5922:
5919:
5917:
5914:
5912:
5909:
5908:
5906:
5900:
5894:
5891:
5889:
5886:
5884:
5881:
5879:
5876:
5874:
5873:Reed reaction
5871:
5869:
5866:
5864:
5861:
5859:
5856:
5854:
5851:
5849:
5846:
5844:
5841:
5839:
5836:
5834:
5831:
5829:
5826:
5824:
5821:
5819:
5816:
5814:
5811:
5809:
5806:
5804:
5801:
5799:
5796:
5794:
5791:
5790:
5788:
5784:bond forming
5780:
5770:
5767:
5765:
5762:
5760:
5757:
5755:
5752:
5750:
5747:
5745:
5742:
5740:
5737:
5735:
5732:
5730:
5727:
5725:
5722:
5720:
5717:
5715:
5712:
5710:
5707:
5705:
5702:
5700:
5697:
5695:
5692:
5690:
5689:Cope reaction
5687:
5685:
5682:
5680:
5677:
5675:
5672:
5670:
5667:
5666:
5664:
5660:
5654:
5651:
5649:
5646:
5644:
5641:
5639:
5636:
5634:
5631:
5629:
5626:
5624:
5621:
5620:
5618:
5616:
5612:
5606:
5603:
5601:
5598:
5596:
5593:
5591:
5588:
5586:
5583:
5581:
5578:
5576:
5573:
5571:
5568:
5566:
5563:
5561:
5558:
5556:
5553:
5551:
5548:
5546:
5543:
5541:
5538:
5536:
5533:
5531:
5528:
5526:
5523:
5521:
5518:
5516:
5513:
5511:
5508:
5506:
5503:
5501:
5498:
5496:
5493:
5491:
5488:
5486:
5483:
5481:
5478:
5476:
5473:
5471:
5468:
5466:
5463:
5461:
5458:
5456:
5453:
5451:
5448:
5446:
5443:
5441:
5438:
5436:
5433:
5431:
5428:
5426:
5423:
5421:
5418:
5416:
5413:
5411:
5408:
5406:
5403:
5401:
5400:Nef synthesis
5398:
5396:
5393:
5391:
5388:
5386:
5383:
5381:
5378:
5376:
5375:Methylenation
5373:
5371:
5368:
5366:
5363:
5361:
5358:
5356:
5353:
5351:
5348:
5346:
5343:
5341:
5338:
5336:
5333:
5331:
5328:
5326:
5323:
5321:
5318:
5316:
5313:
5311:
5308:
5306:
5303:
5301:
5298:
5296:
5293:
5291:
5288:
5286:
5283:
5281:
5278:
5276:
5273:
5271:
5268:
5266:
5263:
5261:
5258:
5256:
5253:
5251:
5248:
5246:
5245:Heck reaction
5243:
5241:
5238:
5236:
5233:
5231:
5228:
5226:
5223:
5221:
5218:
5216:
5213:
5211:
5208:
5206:
5203:
5201:
5198:
5196:
5193:
5191:
5188:
5186:
5183:
5181:
5178:
5176:
5173:
5171:
5168:
5166:
5163:
5161:
5158:
5156:
5153:
5151:
5148:
5146:
5143:
5141:
5138:
5136:
5133:
5131:
5128:
5126:
5123:
5121:
5118:
5116:
5113:
5111:
5108:
5106:
5103:
5101:
5098:
5096:
5093:
5091:
5088:
5086:
5083:
5081:
5078:
5076:
5073:
5071:
5068:
5066:
5063:
5061:
5058:
5056:
5053:
5051:
5048:
5046:
5043:
5041:
5038:
5036:
5033:
5031:
5028:
5026:
5023:
5021:
5018:
5016:
5013:
5011:
5008:
5006:
5003:
5001:
4998:
4996:
4993:
4991:
4988:
4986:
4983:
4981:
4978:
4976:
4973:
4971:
4968:
4966:
4963:
4961:
4958:
4956:
4953:
4951:
4948:
4946:
4943:
4941:
4938:
4936:
4933:
4932:
4930:
4926:bond forming
4922:
4918:
4913:
4907:
4904:
4902:
4899:
4897:
4894:
4892:
4891:Y-aromaticity
4889:
4887:
4884:
4882:
4879:
4877:
4876:Walsh diagram
4874:
4872:
4869:
4867:
4864:
4862:
4861:Taft equation
4859:
4857:
4854:
4852:
4849:
4847:
4844:
4842:
4839:
4837:
4834:
4832:
4831:Σ-aromaticity
4829:
4827:
4824:
4822:
4819:
4817:
4814:
4812:
4809:
4807:
4804:
4802:
4799:
4797:
4794:
4792:
4789:
4787:
4784:
4782:
4779:
4777:
4774:
4772:
4769:
4767:
4764:
4762:
4759:
4757:
4756:Marcus theory
4754:
4752:
4749:
4747:
4744:
4742:
4739:
4737:
4734:
4732:
4731:Hückel's rule
4729:
4727:
4724:
4722:
4719:
4717:
4714:
4712:
4709:
4707:
4704:
4702:
4699:
4697:
4694:
4692:
4689:
4687:
4686:Evelyn effect
4684:
4682:
4679:
4677:
4674:
4672:
4669:
4667:
4666:Electron-rich
4664:
4662:
4659:
4657:
4654:
4652:
4649:
4647:
4644:
4642:
4639:
4637:
4634:
4632:
4629:
4627:
4624:
4622:
4619:
4617:
4614:
4612:
4609:
4607:
4604:
4602:
4599:
4597:
4594:
4592:
4589:
4587:
4584:
4582:
4581:Bema Hapothle
4579:
4577:
4574:
4572:
4569:
4567:
4564:
4562:
4559:
4557:
4554:
4552:
4549:
4547:
4544:
4542:
4539:
4537:
4534:
4532:
4529:
4528:
4525:
4519:
4516:
4514:
4511:
4509:
4506:
4504:
4501:
4499:
4496:
4494:
4491:
4489:
4486:
4484:
4481:
4479:
4476:
4474:
4471:
4470:
4467:
4463:
4455:
4450:
4448:
4443:
4441:
4436:
4435:
4432:
4420:
4412:
4411:
4408:
4402:
4399:
4397:
4394:
4392:
4389:
4387:
4383:
4380:
4378:
4375:
4373:
4370:
4368:
4365:
4363:
4360:
4356:
4353:
4352:
4351:
4348:
4346:
4343:
4341:
4340:Deprotonation
4338:
4337:
4335:
4331:
4325:
4322:
4320:
4316:
4313:
4311:
4307:
4304:
4302:
4299:
4297:
4294:
4292:
4288:
4285:
4284:
4282:
4278:
4272:
4269:
4267:
4265:
4260:
4258:
4255:
4253:
4250:
4248:
4245:
4244:
4242:
4240:
4236:
4230:
4227:
4225:
4222:
4220:
4217:
4215:
4212:
4210:
4207:
4206:
4204:
4200:
4194:
4191:
4190:
4188:
4184:
4178:
4175:
4173:
4170:
4168:
4165:
4163:
4160:
4158:
4155:
4154:
4152:
4148:
4138:
4135:
4134:
4132:
4126:
4120:
4117:
4115:
4112:
4110:
4107:
4105:
4102:
4101:
4099:
4095:Sesquiterpene
4093:
4087:
4084:
4082:
4079:
4077:
4074:
4072:
4069:
4067:
4064:
4062:
4059:
4057:
4054:
4052:
4049:
4048:
4046:
4040:
4037:
4033:
4023:
4020:
4018:
4015:
4013:
4010:
4008:
4005:
4003:
4000:
3998:
3995:
3994:
3992:
3988:
3982:
3979:
3978:
3976:
3970:
3964:
3961:
3960:
3958:
3952:
3941:
3938:
3931:
3928:
3921:
3918:
3911:
3908:
3901:
3898:
3891:
3888:
3881:
3878:
3871:
3868:
3861:
3858:
3851:
3848:
3841:
3838:
3831:
3828:
3821:
3818:
3817:
3815:
3804:
3801:
3799:
3795:
3789:
3786:
3784:
3781:
3779:
3776:
3774:
3773:Oleyl alcohol
3771:
3769:
3766:
3764:
3761:
3759:
3756:
3754:
3751:
3750:
3748:
3746:
3738:
3732:
3729:
3727:
3724:
3722:
3719:
3717:
3714:
3712:
3709:
3707:
3704:
3702:
3699:
3697:
3694:
3693:
3691:
3689:
3683:
3677:
3674:
3672:
3669:
3667:
3664:
3663:
3661:
3659:
3655:
3649:
3646:
3644:
3641:
3639:
3636:
3634:
3631:
3629:
3626:
3624:
3621:
3619:
3616:
3614:
3611:
3610:
3608:
3606:
3605:Amyl alcohols
3602:
3587:
3584:
3577:
3573:
3570:
3563:
3560:
3553:
3549:
3546:
3539:
3535:
3532:
3525:
3522:
3520:
3517:
3516:
3514:
3512:
3508:
3502:
3499:
3498:
3496:
3492:
3486:
3483:
3481:
3478:
3477:
3475:
3471:
3460:
3459:Cetyl alcohol
3457:
3450:
3447:
3440:
3437:
3430:
3427:
3420:
3417:
3410:
3407:
3406:
3404:
3400:
3397:
3393:
3387:
3384:
3382:
3379:
3377:
3375:
3371:
3369:
3368:-Amyl alcohol
3367:
3363:
3361:
3359:
3354:
3352:
3349:
3347:
3346:Ethchlorvynol
3344:
3342:
3339:
3337:
3334:
3332:
3329:
3327:
3324:
3322:
3319:
3317:
3314:
3313:
3311:
3309:alcohols (3°)
3305:
3300:
3297:
3295:
3292:
3290:
3287:
3285:
3282:
3280:
3277:
3275:
3274:Cyclopropanol
3272:
3270:
3269:Cyclopentanol
3267:
3265:
3262:
3260:
3257:
3255:
3252:
3250:
3247:
3245:
3242:
3240:
3237:
3235:
3232:
3230:
3227:
3225:
3222:
3220:
3217:
3215:
3212:
3210:
3207:
3205:
3202:
3200:
3198:alcohols (2°)
3194:
3188:
3185:
3183:
3180:
3178:
3175:
3173:
3170:
3168:
3165:
3163:
3160:
3158:
3155:
3153:
3150:
3148:
3145:
3143:
3140:
3138:
3135:
3133:
3130:
3128:
3125:
3123:
3120:
3118:
3115:
3113:
3110:
3108:
3107:Allyl alcohol
3105:
3103:
3100:
3099:
3090:
3087:
3085:
3082:
3080:
3077:
3075:
3072:
3070:
3067:
3065:
3062:
3060:
3057:
3055:
3052:
3050:
3047:
3045:
3042:
3041:
3039:
3024:
3018:
3015:
3013:
3010:
3008:
3005:
3003:
3000:
2998:
2995:
2992:
2989:
2987:
2984:
2981:
2978:
2976:
2973:
2970:
2967:
2966:
2964:
2949:
2943:
2942:1-Nonacosanol
2940:
2937:
2936:1-Octacosanol
2934:
2932:
2929:
2926:
2925:1-Hexacosanol
2923:
2921:
2918:
2915:
2912:
2910:
2907:
2904:
2901:
2899:
2896:
2893:
2890:
2889:
2887:
2872:
2866:
2865:1-Nonadecanol
2863:
2860:
2859:1-Octadecanol
2857:
2855:
2852:
2849:
2848:1-Hexadecanol
2846:
2844:
2841:
2838:
2835:
2833:
2830:
2827:
2824:
2821:
2818:
2815:
2812:
2811:
2809:
2794:
2787:
2784:
2781:
2778:
2776:
2773:
2771:
2768:
2766:
2763:
2761:
2758:
2756:
2753:
2751:
2748:
2746:
2743:
2742:
2740:
2725:
2719:
2717:
2713:
2711:
2708:
2706:
2703:
2702:
2700:
2698:
2694:
2688:
2685:
2683:
2680:
2678:
2676:
2672:
2670:
2668:
2664:
2660:
2658:
2655:
2653:
2650:
2648:
2645:
2643:
2640:
2638:
2635:
2633:
2630:
2628:
2625:
2623:
2620:
2618:
2615:
2613:
2610:
2608:
2605:
2603:
2600:
2598:
2595:
2593:
2590:
2588:
2585:
2583:
2580:
2578:
2575:
2574:
2572:
2570:
2566:
2560:
2557:
2555:
2552:
2550:
2547:
2545:
2542:
2540:
2539:Aminomethanol
2537:
2535:
2532:
2531:
2529:
2527:
2523:
2520:
2517:
2511:
2501:
2498:
2496:
2493:
2492:
2490:
2488:
2484:
2476:
2473:
2472:
2470:
2468:
2465:
2463:
2462:Ethchlorvynol
2460:
2459:
2457:
2455:
2451:
2445:
2442:
2440:
2438:
2434:
2432:
2431:-Amyl alcohol
2430:
2426:
2424:
2421:
2419:
2416:
2414:
2411:
2409:
2406:
2404:
2401:
2399:
2396:
2395:
2393:
2391:
2385:
2382:
2378:
2374:
2367:
2362:
2360:
2355:
2353:
2348:
2347:
2344:
2333:
2329:
2328:
2320:
2315:
2310:
2306:
2305:
2297:
2292:
2287:
2283:
2282:
2274:
2269:
2264:
2260:
2259:
2251:
2246:
2241:
2237:
2236:
2228:
2223:
2218:
2214:
2213:
2205:
2200:
2195:
2191:
2190:
2182:
2177:
2176:
2174:
2167:
2163:
2162:
2154:
2149:
2144:
2140:
2139:
2131:
2126:
2121:
2117:
2116:
2108:
2103:
2102:
2101:Alkylations:
2100:
2099:
2096:
2086:
2082:
2076:
2068:
2064:
2060:
2056:
2052:
2048:
2044:
2037:
2030:
2029:3-540-40203-9
2026:
2022:
2021:
2014:
2006:
2002:
1998:
1994:
1990:
1987:
1986:
1981:
1974:
1966:
1962:
1958:
1954:
1950:
1947:
1946:
1938:
1930:
1926:
1922:
1919:(in German).
1918:
1917:
1912:
1905:
1897:
1893:
1889:
1885:
1879:
1873:
1865:
1861:
1857:
1853:
1849:
1845:
1838:
1831:
1826:
1818:
1816:0-471-58589-0
1812:
1808:
1801:
1794:
1788:
1784:
1783:
1778:
1771:
1764:
1759:
1752:
1747:
1739:
1735:
1731:
1728:
1727:
1707:
1700:
1695:
1680:
1676:
1670:
1662:
1658:
1654:
1650:
1643:
1635:
1631:
1627:
1623:
1622:
1614:
1606:
1600:
1596:
1592:
1588:
1581:
1572:
1564:
1560:
1556:
1552:
1551:
1543:
1536:
1530:
1526:
1525:
1520:
1513:
1511:
1502:
1498:
1492:
1484:
1480:
1476:
1474:9781305577190
1470:
1466:
1459:
1451:
1447:
1442:
1437:
1433:
1429:
1425:
1421:
1417:
1410:
1402:
1396:
1392:
1388:
1384:
1380:
1373:
1365:
1359:
1355:
1351:
1347:
1343:
1336:
1328:
1324:
1320:
1317:
1316:
1308:
1300:
1294:
1290:
1286:
1282:
1278:
1271:
1264:
1260:
1256:
1252:
1246:
1242:
1233:
1230:
1228:
1225:
1222:
1219:
1218:
1212:
1210:
1206:
1196:
1194:
1183:
1179:
1178:
1177:
1175:
1171:
1167:
1163:
1159:
1146:
1142:
1141:
1140:
1138:
1134:
1130:
1129:zinc chloride
1126:
1118:
1114:
1113:
1112:
1110:
1106:
1102:
1098:
1095:
1091:
1078:
1077:2-bromobutane
1074:
1072:
1067:
1063:
1059:
1055:
1052:
1048:
1045:
1042:
1038:
1034:
1031:
1027:
1023:
1019:
1018:
1017:
1015:
1008:
1004:
1000:
999:isomerization
996:
992:
988:
985:
981:
978:
974:
970:
967:
963:
960:
956:
953:
949:
946:
942:
939:
935:
931:
930:
929:
922:
913:
911:
903:
899:
898:
897:
895:
891:
887:
877:
875:
871:
863:
859:
858:
857:
855:
834:
824:
822:
818:
814:
810:
806:
802:
798:
790:
786:
785:
784:
782:
778:
774:
770:
762:
758:
754:
750:
746:
736:
732:
728:
725:
722:
721:
717:
713:
710:
709:
704:
700:
693:
687:
685:
682:
681:
676:
665:
662:
658:
655:
649:
646:
645:
638:
635:
634:
633:Aromatic Ring
629:
625:
620:
617:
614:
611:
610:
607:
603:
600:
597:
596:
591:
583:
581:
577:
576:4,4'-biphenol
573:
565:
561:
557:
555:
551:
534:
530:
529:
528:
517:
513:
512:
511:
509:
508:sulfuric acid
505:
501:
497:
492:
490:
487:
480:(R---X---AlCl
478:
470:
466:
465:
464:
462:
458:
454:
450:
445:
443:
439:
435:
431:
427:
426:alkyl halides
423:
422:aromatic ring
419:
409:
407:
403:
395:
391:
388:
384:
383:
382:
380:
376:
372:
357:
353:
349:
346:
343:
342:
338:
334:
331:
330:
325:
321:
314:
308:
306:
303:
302:
297:
286:
283:
279:
276:
270:
267:
266:
259:
256:
255:
254:Aromatic Ring
250:
246:
241:
238:
235:
232:
231:
228:
224:
221:
218:
217:
212:
201:
197:
195:
191:
187:
183:
182:aromatic ring
179:
175:
172:developed by
171:
168:are a set of
167:
157:
153:
149:
146:
143:
142:
137:
133:
126:
120:
118:
115:
114:
109:
102:
98:
95:
89:
86:
80:
77:
76:
75:Aromatic Ring
71:
67:
62:
59:
56:
53:
52:
49:
45:
42:
39:
38:
33:
30:
19:
6681:Ene reaction
6041:Autoxidation
5902:Degradation
5793:Azo coupling
5570:Ugi reaction
5194:
5170:Ene reaction
4970:Alkynylation
4821:Polyfluorene
4816:Polar effect
4681:Electrophile
4596:Bredt's rule
4566:Baird's rule
4536:Alpha effect
4287:Substitution
4280:Preparations
4263:
4229:Stigmasterol
4224:β-Sitosterol
4022:Polyglycitol
4012:Maltotriitol
3972:Cyclic sugar
3740:Branched and
3648:Pentan-3-ol
3643:Pentan-2-ol
3638:Pentan-1-ol
3373:
3365:
3357:
3264:Cyclohexanol
2916:(lignoceryl)
2909:1-Tricosanol
2832:1-Tridecanol
2788:(pelargonic)
2715:
2674:
2666:
2662:
2657:Ethanolamine
2436:
2428:
2331:
2325:
2308:
2302:
2285:
2279:
2262:
2256:
2239:
2233:
2216:
2210:
2193:
2187:
2175:Acylations:
2165:
2159:
2142:
2136:
2119:
2113:
2094:
2075:
2050:
2046:
2036:
2023:, Springer,
2019:
2013:
1991:(11): 1799.
1988:
1983:
1973:
1948:
1943:
1937:
1920:
1914:
1904:
1887:
1883:
1872:
1847:
1843:
1837:
1825:
1806:
1800:
1781:
1777:March, Jerry
1770:
1758:
1746:
1729:
1724:
1706:
1694:
1682:. Retrieved
1678:
1669:
1652:
1648:
1642:
1625:
1619:
1613:
1586:
1580:
1571:
1554:
1548:
1542:
1523:
1519:March, Jerry
1464:
1458:
1423:
1419:
1409:
1382:
1378:
1372:
1345:
1341:
1335:
1318:
1313:
1307:
1280:
1276:
1270:
1254:
1251:Compt. Rend.
1250:
1245:
1203:Reaction of
1202:
1187:
1155:
1122:
1087:
1070:
1050:
1036:
1021:
1011:
1007:phenanthrene
1002:
990:
976:
927:
907:
883:
867:
853:
830:
800:
797:benzaldehyde
794:
759:catalyst is
742:
731:RXNO:0000045
726:ontology ID
706:Identifiers
660:
651:
642:
631:
606:James Crafts
598:Named after
569:
556:reactions.
547:
526:
496:carbocations
493:
474:
460:
449:nucleophilic
446:
415:
399:
375:ethylbenzene
368:
365:With alkenes
352:RXNO:0000046
347:ontology ID
327:Identifiers
281:
272:
263:
252:
227:James Crafts
219:Named after
178:James Crafts
165:
163:
152:RXNO:0000369
147:ontology ID
139:Identifiers
100:
91:
84:
73:
48:James Crafts
40:Named after
29:
5180:Ethenolysis
4826:Ring strain
4796:Nucleophile
4621:Clar's rule
4561:Aromaticity
4345:Protonation
4262:Nonafluoro-
4209:Cholesterol
4186:Trialcohols
4056:Citronellol
4042:Monoterpene
3954:Deoxy sugar
3742:unsaturated
3429:Isopropanol
3356:Nonafluoro-
3284:Isopropanol
2903:1-Docosanol
2894:(arachidyl)
2826:1-Dodecanol
2820:1-Undecanol
1951:(84): 692.
1923:: 147–156.
1385:: 733–752.
1348:: 707–731.
1137:rhodamine B
1133:fluorescein
1044:hydrocarbon
1026:cyclohexene
995:dehydration
833:arenium ion
781:acylium ion
777:carbocation
771:group, the
678:Conditions
486:carbocation
402:solid acids
299:Conditions
111:Conditions
7743:Categories
7464:Ozonolysis
6991:Annulation
6341:Ozonolysis
4460:Topics in
4306:Hydrolysis
4291:haloalkane
4219:Lanosterol
4214:Ergosterol
4150:Dialcohols
4119:Patchoulol
3920:Galactitol
3850:Erythritol
3726:Tridecanol
3548:Erythritol
3449:1-Pentanol
3259:3-Pentanol
3254:2-Pentanol
3224:3-Heptanol
3219:2-Heptanol
3177:Tryptophol
2982:(lacceryl)
2892:1-Icosanol
2839:(myristyl)
2822:(hendecyl)
2775:1-Heptanol
2765:1-Pentanol
2755:1-Propanol
2710:Isobutanol
2444:Tryptophol
2418:Isobutanol
2398:1-Propanol
1684:26 January
1679:orgsyn.org
1604:3527306730
1298:0471264180
1277:Org. React
1238:References
1205:chloroform
1125:resorcinol
1060:variation
934:Clemmensen
757:Lewis acid
550:reversible
510:catalyst.
440:, such as
438:Lewis acid
418:alkylation
207:Alkylation
186:alkylation
6978:reactions
6493:reactions
5988:reactions
5904:reactions
5786:reactions
4928:reactions
4333:Reactions
4315:Hydration
4128:Diterpene
4114:Nerolidol
4104:Bisabolol
4086:Terpineol
3940:Volemitol
3731:Undecanol
3685:Saturated
3586:Volemitol
3439:1-Butanol
3249:2-Octanol
3244:2-Nonanol
3234:3-Hexanol
3229:2-Hexanol
3209:2-Butanol
3196:Secondary
3028:saturated
2953:saturated
2905:(behenyl)
2876:saturated
2861:(stearyl)
2814:1-Decanol
2798:saturated
2786:1-Nonanol
2780:1-Octanol
2770:1-Hexanol
2760:1-Butanol
2729:saturated
2067:0022-3263
2031:, p. 175.
1491:cite book
1483:915490547
1041:saturated
1039:(1936) a
886:aldehydes
854:catalytic
745:acylation
622:Reaction
586:Acylation
523:Mechanism
243:Reaction
190:acylation
170:reactions
64:Reaction
4871:Vinylogy
4541:Annulene
4488:Reagents
4419:Category
4193:Glycerol
4130:alcohols
4109:Farnesol
4097:alcohols
4081:Rhodinol
4066:Linalool
4061:Geraniol
4044:alcohols
4002:Lactitol
3997:Maltitol
3981:Inositol
3974:alcohols
3956:alcohols
3910:Sorbitol
3900:Mannitol
3870:Arabitol
3860:Threitol
3840:Glycerol
3576:Sorbitol
3572:Mannitol
3552:Threitol
3534:Glycerol
3501:Glycerol
3409:Methanol
3307:Tertiary
2993:(geddyl)
2828:(lauryl)
2816:(capric)
2782:(capryl)
2745:Methanol
2718:-Butanol
2526:Methanol
2516:alcohols
2500:Methanol
2373:Alcohols
2130:"Durene"
1864:23852649
1779:(2007),
1521:(2007),
1450:20485588
1426:(6): 6.
1215:See also
1094:xanthene
1066:graphite
769:carbonyl
684:Catalyst
477:tertiary
434:epoxides
305:Catalyst
117:Catalyst
4531:A value
4310:epoxide
4202:Sterols
4071:Menthol
4051:Borneol
4007:Isomalt
3963:Fucitol
3890:Xylitol
3880:Ribitol
3721:Octanol
3716:Nonanol
3701:Decanol
3562:Xylitol
3419:Ethanol
2927:(ceryl)
2750:Ethanol
2697:Butanol
2652:Ethanol
2569:Ethanol
2514:Primary
2475:Ethanol
2408:Ethanol
1993:Bibcode
1953:Bibcode
1441:2870981
1073:-xylene
982:In the
971:In the
910:phenone
894:glyoxal
890:mesityl
835:by AlCl
801:in situ
692:Zeolite
406:zeolite
371:alkenes
313:Zeolite
125:Zeolite
4319:alkene
4137:Phytol
3930:Iditol
3162:Prenol
2083:
2065:
2027:
1862:
1813:
1789:
1655:: 12.
1601:
1557:: 90.
1531:
1481:
1471:
1448:
1438:
1397:
1360:
1321:: 73.
1295:
1261:&
1105:thymol
773:ketone
506:using
430:enones
420:of an
4076:Nerol
2322:(PDF)
2299:(PDF)
2276:(PDF)
2265:: 227
2253:(PDF)
2230:(PDF)
2207:(PDF)
2184:(PDF)
2156:(PDF)
2133:(PDF)
2110:(PDF)
1283:: 1.
1172:or a
1162:arene
1075:with
1056:In a
1028:with
936:or a
4264:tert
3374:tert
3366:tert
3358:tert
2518:(1°)
2437:tert
2429:tert
2334:: 28
2311:: 43
2288:: 17
2242:: 16
2196:: 29
2168:: 26
2145:: 32
2122:: 38
2081:ISBN
2063:ISSN
2025:ISBN
1949:2007
1860:PMID
1811:ISBN
1787:ISBN
1686:2019
1599:ISBN
1529:ISBN
1501:link
1497:link
1479:OCLC
1469:ISBN
1446:PMID
1395:ISBN
1358:ISBN
1293:ISBN
1263:1450
1259:1392
1156:The
1107:and
1097:dyes
1092:and
1084:Dyes
1049:The
1020:The
997:and
989:The
957:The
950:The
943:The
819:and
811:and
696:AlCl
432:and
317:AlCl
176:and
164:The
129:AlCl
4384:of
4317:of
4308:of
4289:of
3810:— C
3034:— C
2959:— C
2882:— C
2804:— C
2735:— C
2219:: 1
2055:doi
2001:doi
1961:doi
1925:doi
1892:doi
1852:doi
1734:doi
1657:doi
1630:doi
1591:doi
1559:doi
1436:PMC
1428:doi
1387:doi
1350:doi
1323:doi
1285:doi
1190:SeO
1001:of
872:or
751:.
724:RSC
345:RSC
196:.
145:RSC
7745::
3942:(C
3932:(C
3922:(C
3912:(C
3902:(C
3892:(C
3882:(C
3872:(C
3862:(C
3852:(C
3842:(C
3832:(C
3822:(C
3588:(C
3578:(C
3574:,
3564:(C
3554:(C
3550:,
3540:(C
3536:,
3526:(C
3463:16
3461:(C
3451:(C
3441:(C
3431:(C
3421:(C
3411:(C
3036:49
3032:40
2961:39
2957:30
2884:29
2880:20
2806:19
2802:10
2332:56
2330:.
2324:.
2307:.
2301:.
2284:.
2278:.
2263:80
2261:.
2255:.
2240:12
2238:.
2232:.
2217:20
2215:.
2209:.
2194:20
2192:.
2186:.
2164:.
2158:.
2143:10
2141:.
2135:.
2120:29
2118:.
2112:.
2061:.
2051:45
2049:.
2045:.
1999:.
1989:84
1982:.
1959:.
1921:23
1913:.
1888:58
1886:.
1880::
1858:.
1848:52
1846:.
1730:57
1719:,6
1715:,4
1677:.
1653:13
1651:.
1626:34
1624:.
1597:.
1555:32
1553:.
1509:^
1493:}}
1489:{{
1477:.
1444:.
1434:.
1422:.
1418:.
1393:.
1381:.
1356:.
1344:.
1291:.
1279:.
1257::
1255:84
1253:,
1139::
1111::
979:).
912:.
694:,
582:.
381::
315:,
127:,
4453:e
4446:t
4439:v
3946:)
3944:7
3936:)
3934:6
3926:)
3924:6
3916:)
3914:6
3906:)
3904:6
3896:)
3894:5
3886:)
3884:5
3876:)
3874:5
3866:)
3864:4
3856:)
3854:4
3846:)
3844:3
3836:)
3834:2
3826:)
3824:1
3812:7
3808:1
3806:C
3592:)
3590:7
3582:)
3580:6
3568:)
3566:5
3558:)
3556:4
3544:)
3542:3
3530:)
3528:2
3465:)
3455:)
3453:5
3445:)
3443:4
3435:)
3433:3
3425:)
3423:2
3415:)
3413:1
3030:C
2955:C
2878:C
2800:C
2737:9
2733:1
2731:C
2716:n
2675:N
2667:N
2665:,
2663:N
2365:e
2358:t
2351:v
2336:.
2313:.
2309:4
2290:.
2286:5
2267:.
2244:.
2221:.
2198:.
2170:.
2166:8
2147:.
2124:.
2069:.
2057::
2007:.
2003::
1995::
1967:.
1963::
1955::
1931:.
1927::
1898:.
1894::
1866:.
1854::
1819:.
1740:.
1736::
1721:′
1717:′
1713:′
1688:.
1663:.
1659::
1636:.
1632::
1607:.
1593::
1566:.
1561::
1503:)
1485:.
1452:.
1430::
1424:6
1403:.
1389::
1383:2
1366:.
1352::
1346:2
1329:.
1325::
1319:1
1301:.
1287::
1281:3
1265:.
1192:2
1071:p
968:.
940:.
850:3
846:3
841:3
837:4
765:3
698:3
667:+
653:↓
640:+
482:3
461:t
319:3
288:+
274:↓
261:+
131:3
93:↓
82:+
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.