279:. Because of the newness of the field, DFC has only recently been used to investigate disease states, but since 2012 each of these three diseases has been shown to be correlated to dynamic temporal characteristics in functional connectivity. Most of these differences are related to the amount of time that is spent in different transient states. Patients with Schizophrenia have less frequent state changes than healthy patients, and this result has led to the suggestion that the disease is related to patients being stuck in certain brain states where the brain is unable to respond quickly to different queues. Also, a study in the visual sensory network showed that schizophrenia subjects spent more time than the healthy subjects in a state in which the connectivity between the middle temporal gyrus and other regions of the visual sensory network is highly negative. Studies with Alzheimer's disease have shown that patients with this ailment have altered network connectivity as well as altered time spent in the networks that are present. The observed correlation between DFC and disease does not imply that the changes in DFC are the cause of any of these diseases, but information from DFC analysis may be used to better understand the effects of the disease and to more quickly and accurately diagnose them.
119:
scans is the length of the sliding window. The defined window is then moved a certain number of scans forward in time and additional analysis is performed. The movement of the window is usually referenced in terms of the degree of overlap between adjacent windows. One of the principle benefits of sliding window analysis is that almost any steady state analysis can also be performed using sliding window if the window length is sufficiently large. Sliding window analysis also has a benefit of being easy to understand and in some ways easier to interpret. As the most common method of analysis, sliding window analysis has been used in many different ways to investigate a variety of different characteristics and implications of DFC. In order to be accurately interpreted, data from sliding window analysis generally must be compared between two different groups. Researchers have used this type of analysis to show different DFC characteristics in diseased and healthy patients, high and low performers on cognitive tasks, and between large scale brain states.
101:
dynamic functional connectivity has shown that far from being completely static, the functional networks of the brain fluctuate on the scale of seconds to minutes. These changes are generally seen as movements from one short term state to another, rather than continuous shifts. Many studies have shown reproducible patterns of network activity that move throughout the brain. These patterns have been seen in both animals and humans, and are present at only certain points during a scanner session. In addition to showing transient brain states, DFC analysis has shown a distinct hierarchical organization of the networks of the brain. Connectivity between bilaterally symmetric regions is the most stable form of connectivity in the brain, followed by other regions with direct anatomical connections. Steady state functional connectivity networks exist and have
184:
is heavily influenced by the ratio of oxygenated and deoxygenated blood. Since active neurons require more energy than resting neurons, changes in this contrast is traditionally interpreted an indirect measure of neural activity. Because of its indirect nature, fMRI data in DFC studies could be criticized as potentially being a reflection of non neural information. This concern has been alleviated recently by the observed correlation between fMRI DFC and simultaneously acquired electrophysiology data. Battaglia and colleagues have tried to address those controversies, linking dynamic functional connectivity to causality or effective connectivity. The scientists claim indeed that dynamic effective connectivity can emerge from transitions in the collective organization of coherent neural activity.
258:
on the magnitude of activation in brain regions as a predictor of performance, but recent research has shown that correlation between networks as measured with sliding window analysis is an even stronger predictor of performance. Individual differences in functional connectivity variability (FCV) across sliding windows within fMRI scans have been shown to correlate with the tendency to attend to pain. The degree to which a subject is mind wandering away from a sensory stimulus has also been related to FCV.
74:
236:(MEG) can be used to measure the magnetic fields produced by electrical activity in the brain. MEG has high temporal resolution and has generally higher spatial resolution than EEG. Resting state studies with MEG are still limited by spatial resolution, but the modality has been used to show that resting state networks move through periods of low and high levels of correlation. This observation is consistent with the results seen in other DFC studies such as DFC activation pattern analysis.
225:(EEG) has also been used in humans to both validate and interpret observations made in DFC. EEG has poor spatial resolution because it is only able to acquire data on the surface of the scalp, but it is reflective of broad electrical activity from many neurons. EEG has been used simultaneously with fMRI to account for some of the inter scan variance in FC. EEG has also been used to show that changes in FC are related to broad brain states observed in EEG.
245:
coefficients were typically < 0.05. These functional connections were found to be plastic – changing the correlation for a conditioning period of Ts (typically a few minutes), by means of spike-triggered sensory stimulations, induced short-term (typically < Ts) lasting changes of the connections. The pre-post conditioning strengthening of a functional connection was typically equal to the square root of its pre-during conditioning strengthening.
128:
probably reflects some of the constant processes of the brain. Repeating patterns of network information have been suggested to account for 25–50% of the variance in fMRI BOLD data. These patterns of activity have primarily been seen in rats as a propagating wave of synchronized activity along the cortex. These waves have also been shown to be related to underlying neural activity, and has been shown to be present in humans as well as rats.
211:
180:
analysis. Some researchers have proposed that the variability in functional connectivity in fMRI studies is consistent with the variability that one would expect from simply analyzing random data. This complaint that DFC may reflect only noise has been recently lessened by the observation of electrical basis to fMRI DFC data and behavioral relevance of DFC characteristics.
92:
tasks, sleep, and learning. These changes often occur within the same individual and are clearly relevant to behavior. DFC has now been investigated in a variety of different contexts with many analysis tools. It has been shown to be related to both behavior and neural activity. Some researchers believe that it may be heavily related to high level thought or consciousness.
70:, and spatial grouping based on temporal similarities. These methods have been used to show that functional connectivity is related to behavior in a variety of different tasks, and that it has a neural basis. These methods assume the functional connections in the brain remain constant in a short time over a task or period of data collection.
137:
the peaks). These few points contain a great portion of the information pertaining functional connectivity, because it has been demonstrated, that despite the tremendous reduction on the data size (> 95%), it compares very well with inferences of functional connectivity obtained with standard methods which uses the full signal.
249:
potassium channels on spines, thus weakening or strengthening connectivity, respectively . For example, dopamine D1 receptor and/or noradrenergic beta-1 receptor stimulation on spines can increase cAMP-PKA-calcium signaling to open HCN, KCNQ2, and/or SK channels to rapidly weaken a connection, e.g. as occurs during stress .
257:
DFC has been shown to be significantly related to human performance, including vigilance and aspects of attention. It has been proposed and supported that the network behavior immediately prior to a task onset is a strong predictor of performance on that task. Traditionally, fMRI studies have focused
248:
Dynamic
Functional Connectivity studied using fMRI may be related to a phenomenon previously discovered in macaque prefrontal cortex termed Dynamic Network Connectivity, whereby arousal mechanisms rapidly alter the strength of glutamate synaptic connections onto dendritic spines by opening or closing
183:
In addition to complaints that DFC may be a product of scanner noise, observed DFC could be criticized based on the indirect nature of fMRI which is used to observe it. fMRI data is collected by quickly acquiring a sequence of MRI images in time using echo planar imaging. The contrast in these images
170:
has become one of the most common methods of network generation in steady state functional connectivity. ICA divides fMRI signal into several spatial components that have similar temporal patterns. More recently, ICA has been used to divide fMRI data into different temporal components. This has been
140:
The large information content of these few points is consistent with the results of
Petridou et al. who demonstrated he contribution of these "spontaneous events" to the correlation strength and power spectra of the slow spontaneous fluctuations by deconvolving the task hemodynamic response function
136:
Departing from the traditional approaches, recently an efficient method was introduced to analyze rapidly changing functional activations patterns which transforms the fMRI BOLD data into a point process. This is achieved by selecting for each voxel the points of inflection of the BOLD signal (i.e.,
91:
Studies that showed brain state dependent changes in functional connectivity were the first indicators that temporal variation in functional connectivity may be significant. Several studies in the mid-2000s examined the changes in FC that were related to a variety of different causes such as mental
206:
Correlation between DFC and electrophysiology has led some scientists to suggest that DFC could reflect hemodynamic results of dynamic network behavior that has been seen in single cell analysis of neuron populations. Although hemodynamic response is too slow to reflect a one-to-one correspondence
179:
Several researchers have argued that DFC may be a simple reflection of analysis, scanner, or physiological noise. Noise in fMRI can arise from a variety of different factors including heart beat, changes in the blood brain barrier, characteristics of the acquiring scanner, or unintended effects of
53:
which looks for physical connections in the brain, functional connectivity is related to similar patterns of activation in different brain regions regardless of the apparent physical connectedness of the regions. This type of connectivity was discovered in the mid-1990s and has been seen primarily
27:
changes over a short time. Dynamic functional connectivity is a recent expansion on traditional functional connectivity analysis which typically assumes that functional networks are static in time. DFC is related to a variety of different neurological disorders, and has been suggested to be a more
192:
fMRI is the primary means of investigating DFC. This presents unique challenges because fMRI has fairly low temporal resolution, typically 0.5 Hz, and is only an indirect measure of neural activity. The indirect nature of fMRI analysis suggests that validation is needed to show that findings
118:
Sliding window analysis is the most common method used in the analysis of functional connectivity, first introduced by
Sakoglu and Calhoun in 2009, and applied to schizophrenia. Sliding window analysis is performed by conducting analysis on a set number of scans in an fMRI session. The number of
244:
Single-unit recording were used in order to explore the extent, strength and plasticity of functional connectivity between individual cortical neurons in cats and monkeys. Such studies revealed correlated activity at various time scales. At the fastest time scale, that of 1 – 20 ms, correlation
127:
One of the first methods ever used to analyze DFC was pattern analysis of fMRI images to show that there are patterns of activation in spatially separated brain regions that tend to have synchronous activity. It has become clear that there is a spatial and temporal periodicity in the brain that
100:
Because DFC is such a new field, much of the research related to it is conducted to validate the relevance of these dynamic changes rather than explore their implications; however, many critical findings have been made that help the scientific community better understand the brain. Analysis of
157:
has been used to conduct DFC analysis that has validated the existence of DFC by showing its significant changes in time. This same method has recently been used to investigate some of the dynamic characteristics of accepted networks. For example, time frequency analysis has shown that the
404:
Hutchison, R. M.; Womelsdorf, T.; Allen, E. A.; Bandettini, P. A.; Calhoun, V. D.; Corbetta, M.; Della Penna, S.; Duyn, J. H.; Glover, G. H.; Gonzalez-Castillo, J.; Handwerker, D. A.; Keilholz, S.; Kiviniemi, V.; Leopold, D. A.; De
Pasquale, F.; Sporns, O.; Walter, M.; Chang, C. (2013).
152:
has been proposed as an analysis method that is capable of overcoming many of the challenges associated with sliding windows. Unlike sliding window analysis, time frequency analysis allows the researcher to investigate both frequency and amplitude information simultaneously. The
105:, but have less temporal stability than the anatomical networks. Finally, some functional networks are fleeting enough to only be seen with DFC analysis. These networks also possess physiological relevance but are much less temporally stable than the other networks in the brain.
454:
Esposito, F.; Bertolino, A.; Scarabino, T.; Latorre, V.; Blasi, G.; Popolizio, T.; Tedeschi, G.; Cirillo, S.; Goebel, R.; Di Salle, F. (2006). "Independent component model of the default-mode brain function: Assessing the impact of active thinking".
81:
above is one example of a brain network seen using steady state connectivity. This network is fairly stable in time, but it has been shown to have a variable relationship with other networks, and to vary slightly in its own characteristics in time.
1916:
Jones, D. T.; Vemuri, P.; Murphy, M. C.; Gunter, J. L.; Senjem, M. L.; Machulda, M. M.; Przybelski, S. A.; Gregg, B. E.; Kantarci, K.; Knopman, D. S.; Boeve, B. F.; Petersen, R. C.; Jack Jr, C. R. (2012). He, Yong (ed.).
266:
One of the principal motivations of DFC analysis is to better understand, detect and treat neurological diseases. Static functional connectivity has been shown to be significantly related to a variety of diseases such as
1693:
Frostig, R.D., Gottlieb, Y., Vaadia, E. & Abeles, M. The effects of stimuli on the activity and functional connectivity of local neuronal groups in the cat auditory cortex. Brain Res. 272, 211-221 (1983)
1851:
Damaraju, E.; J. Turner; A. Preda; T. Erp Van; D. Mathalon; J.M. Ford; S. Potkin; V.D. Calhoun (2012). "Static and dynamic functional network connectivity during resting state in schizophrenia".
1703:
Aertsen, A., et al. Neural interactions in the frontal cortex of a behaving monkey: signs of dependence on stimulus context and behavioral state. J.Hirnforsch. 32, 735-743 (1991)
1721:
Arnsten, A.F.T., Wang, M., Paspalas, C.D. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 76, 223-39 (2012)
1739:
Datta, D., Yang, S. et al., Key Roles of CACNA1C/Cav1.2 and CALB1/Calbindin in
Prefrontal Neurons Altered in Cognitive Disorders. JAMA Psychiatry. epub May 22 (2024)
50:
348:
Biswal, B.; Zerrin Yetkin, F. Z.; Haughton, V. M.; Hyde, J. S. (1995). "Functional connectivity in the motor cortex of resting human brain using echo-planar MRI".
746:
Sakoglu, U.; Michael, A. M.; Calhoun, V. D. (2009). "Classification of schizophrenia patients vs healthy controls with dynamic functional network connectivity".
711:
Sakoglu, U.; Calhoun, V. D. (2009). "Temporal
Dynamics of Functional Network Connectivity at Rest: A Comparison of Schizophrenia Patients and Healthy Controls".
1712:
Ahissar, E., et al. Dependence of cortical plasticity on correlated activity of single neurons and on behavioral context. Science 257, 1412-1415 (1992)
218:
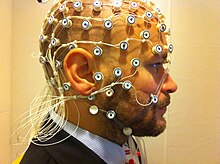
caps like the one above are often used simultaneously with fMRI in order to capture information about the electrical signals underlying the BOLD signal.
171:
termed temporal ICA and it has been used to plot network behavior that accounts for 25% of variability in the correlation of anatomical nodes in fMRI.
1434:
Thompson, G. J.; Magnuson, M. E.; Merritt, M. D.; Schwarb, H.; Pan, W. J.; McKinley, A.; Tripp, L. D.; Schumacher, E. H.; Keilholz, S. D. (2013).
1436:"Short-time windows of correlation between large-scale functional brain networks predict vigilance intraindividually and interindividually"
1730:
Arnsten, A.F.T., Wang, M., D'Esposito. Dynamic
Network Connectivity: from monkeys to humans. Front Hum Neurosci. 18, 1353043 (2024)
36:
whose discovery was motivated by the observation of temporal variability in the rising field of steady state connectivity research.
1483:
Battaglia, Demian; Witt, Annette; Wolf, Fred; Geisel, Theo (2012). "Dynamic effective connectivity of inter-areal brain circuits".
207:
with neural network dynamics, it is plausible that DFC is a reflection of the power of some frequencies of electrophysiology data.
1056:"The voxel-wise functional connectome can be efficiently derived from co-activations in a sparse spatio-temporal point-process"
141:
from the rest data. Subsequently, similar principles were successfully applied under the name of co-activation patterns (CAP).
49:
Functional connectivity refers to the functionally integrated relationship between spatially separated brain regions. Unlike
1107:"Periods of rest in fMRI contain individual spontaneous events which are related to slowly fluctuating spontaneous activity"
1867:
1651:
Mehrkanoon, S; Breakspear, M; Boonstra, T. W. (2014). "Low-dimensional dynamics of resting-state cortical activity".
687:"Dynamic windowing reveals task-modulation of functional connectivity in schizophrenia patients vs healthy controls"
498:
Horovitz, S. G.; Fukunaga, M.; De Zwart, J. A.; Van
Gelderen, P.; Fulton, S. C.; Balkin, T. J.; Duyn, J. H. (2008).
783:"A method for evaluating dynamic functional network connectivity and task-modulation: application to schizophrenia"
1808:
Kucyi, A; Davis, K. D. (2014). "Dynamic functional connectivity of the default mode network tracks daydreaming".
1502:
Thompson, G. J.; Merritt, M. D.; Pan, W. J.; Magnuson, M. E.; Grooms, J. K.; Jaeger, D.; Keilholz, S. D. (2013).
1105:
Petridou, Natalia; Gaudes, César
Caballero; Dryden, Ian L.; Francis, Susan T.; Gowland, Penny A. (2013-06-01).
830:
Majeed, W.; Magnuson, M.; Hasenkamp, W.; Schwarb, H.; Schumacher, E. H.; Barsalou, L.; Keilholz, S. D. (2011).
167:
1866:
Sendi, M.S.E.; Godfrey D. Pearlson; Daniel H. Mathalon; J.M. Ford; A. Preda; T. Erp Van; V.D. Calhoun (2021).
67:
32:, but DFC has also been observed with several other mediums. DFC is a recent development within the field of
500:"Low frequency BOLD fluctuations during resting wakefulness and light sleep: A simultaneous EEG-fMRI study"
59:
1164:"Time-varying functional network information extracted from brief instances of spontaneous brain activity"
879:
Tagliazucchi, Enzo; Balenzuela, Pablo; Fraiman, Daniel; Montoya, Pedro; Chialvo, Dante R. (2011-01-20).
979:
Tagliazucchi, Enzo; Carhart-Harris, Robin; Leech, Robert; Nutt, David; Chialvo, Dante R. (2014-11-01).
672:
Detection of task transitions on 45mins long continuous muli task runs using whole brain connectivity
309:
1751:"Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks"
881:"Spontaneous BOLD event triggered averages for estimating functional connectivity at resting state"
1384:"Cognitive and default-mode resting state networks: Do male and female brains "rest" differently?"
547:
Bassett, D. S.; Wymbs, N. F.; Porter, M. A.; Mucha, P. J.; Carlson, J. M.; Grafton, S. T. (2011).
149:
102:
33:
24:
781:
Sakoglu, U.; Pearlson, G. D.; Kiehl, K. A.; Wang, Y. M.; Michael, A. M.; Calhoun, V. D. (2010).
686:
304:
276:
233:
222:
1981:
163:
28:
accurate representation of functional brain networks. The primary tool for analyzing DFC is
1930:
1762:
1175:
1002:
930:"Criticality in large-scale brain fMRI dynamics unveiled by a novel point process analysis"
570:
159:
78:
1282:"Introducing co-activation pattern metrics to quantify spontaneous brain network dynamics"
8:
1054:
Tagliazucchi, Enzo; Siniatchkin, Michael; Laufs, Helmut; Chialvo, Dante R. (2016-01-01).
268:
1934:
1766:
1553:"Dynamic BOLD functional connectivity in humans and its electrophysiological correlates"
1179:
1006:
928:
Tagliazucchi, Enzo; Balenzuela, Pablo; Fraiman, Daniel; Chialvo, Dante R. (2012-01-01).
574:
1953:
1918:
1898:
1833:
1785:
1750:
1676:
1628:
1603:
1579:
1552:
1528:
1503:
1460:
1435:
1408:
1383:
1359:
1334:
1306:
1281:
1257:
1231:"Decomposition of spontaneous brain activity into distinct fMRI co-activation patterns"
1230:
1206:
1163:
1139:
1106:
1082:
1055:
1031:
992:
980:
956:
929:
905:
880:
856:
831:
807:
782:
763:
728:
670:
Gonzalez, Castillo; J. Wu; P. Robinson; M. Handwerker; D. Inati; S. Bandettini (2012).
647:
622:
593:
560:
548:
524:
499:
480:
468:
431:
406:
373:
330:
63:
1868:"Multiple overlapping dynamic patterns of the visual sensory network in schizophrenia"
759:
724:
1958:
1902:
1890:
1825:
1821:
1790:
1668:
1633:
1619:
1584:
1533:
1519:
1465:
1413:
1364:
1350:
1311:
1297:
1280:
Chen, Jingyuan E.; Chang, Catie; Greicius, Michael D.; Glover, Gary H. (2015-05-01).
1262:
1211:
1193:
1144:
1126:
1087:
1036:
1018:
961:
910:
861:
847:
812:
652:
598:
529:
472:
436:
422:
365:
322:
154:
1837:
1680:
767:
732:
484:
1948:
1938:
1882:
1865:
1817:
1780:
1770:
1660:
1623:
1615:
1574:
1564:
1523:
1515:
1455:
1447:
1403:
1395:
1354:
1346:
1301:
1293:
1252:
1242:
1201:
1183:
1134:
1118:
1077:
1067:
1026:
1010:
951:
941:
900:
892:
851:
843:
802:
794:
755:
720:
642:
634:
588:
578:
519:
511:
464:
426:
418:
357:
334:
314:
377:
1943:
1886:
981:"Enhanced repertoire of brain dynamical states during the psychedelic experience"
896:
1335:"Time–frequency dynamics of resting-state brain connectivity measured with fMRI"
1382:
Weissman-Fogel, I.; Moayedi, M.; Taylor, K. S.; Pope, G.; Davis, K. D. (2010).
1330:
832:"Spatiotemporal dynamics of low frequency BOLD fluctuations in rats and humans"
623:"Spatiotemporal dynamics of low frequency fluctuations in BOLD fMRI of the rat"
1664:
1551:
Tagliazucchi, E; von Wegner, F; Morzelewski, A; Brodbeck, V; Laufs, H (2012).
798:
73:
1975:
1569:
1247:
1197:
1130:
1072:
1022:
946:
272:
1775:
1188:
583:
318:
1962:
1894:
1829:
1794:
1672:
1637:
1588:
1537:
1469:
1417:
1368:
1315:
1266:
1215:
1148:
1091:
1040:
965:
914:
865:
816:
656:
602:
533:
476:
440:
361:
326:
369:
1550:
295:
Friston, Karl (2011). "Functional and
Effective Connectivity: a review".
638:
407:"Dynamic functional connectivity: Promise, issues, and interpretations"
1504:"Neural correlates of time-varying functional connectivity in the rat"
1451:
1399:
1122:
1014:
515:
669:
927:
878:
497:
1850:
1053:
978:
193:
from fMRI are actually relevant and reflective of neural activity.
997:
565:
403:
549:"Dynamic reconfiguration of human brain networks during learning"
453:
1919:"Non-Stationarity in the "Resting Brain's" Modular Architecture"
210:
1381:
347:
1433:
829:
1604:"EEG correlates of time-varying BOLD functional connectivity"
787:
Magnetic Resonance Materials in Physics, Biology and Medicine
1650:
1602:
Chang, C; Liu, Z; Chen, M. C.; Liu, X; Duyn, J. H. (2013).
1104:
55:
29:
1501:
215:
166:
is not constant in time but rather is a temporary state.
780:
674:. Biennial Resting state Conference. Magdeburg, Germany.
1915:
1482:
1279:
546:
620:
62:. Functional connectivity is usually measured during
1229:
Liu, Xiao; Chang, Catie; Duyn, Jeff H. (2013-01-01).
745:
66:and is typically analyzed in terms of correlation,
1748:
621:Majeed, W.; Magnuson, M.; Keilholz, S. D. (2009).
86:
1973:
1749:Kucyi, A; Salomons, T. V.; Davis, K. D. (2013).
678:
95:
1755:Proceedings of the National Academy of Sciences
1601:
1168:Proceedings of the National Academy of Sciences
553:Proceedings of the National Academy of Sciences
710:
684:
174:
1724:
1715:
1697:
1687:
739:
704:
1429:
1427:
1228:
1853:American College of Neuropsychopharmacology
1733:
1328:
774:
399:
397:
395:
393:
391:
389:
387:
1807:
1952:
1942:
1801:
1784:
1774:
1742:
1706:
1627:
1578:
1568:
1527:
1495:
1459:
1424:
1407:
1358:
1305:
1256:
1246:
1205:
1187:
1138:
1081:
1071:
1030:
996:
955:
945:
904:
855:
806:
646:
616:
614:
612:
592:
582:
564:
523:
430:
308:
187:
131:
23:) refers to the observed phenomenon that
1844:
384:
209:
72:
1162:Liu, Xiao; Duyn, Jeff H. (2013-03-12).
1161:
823:
663:
447:
294:
196:
39:
1974:
609:
540:
491:
288:
239:
122:
108:
44:
1909:
1375:
1322:
627:Journal of Magnetic Resonance Imaging
341:
261:
685:Sakoglu, U.; Calhoun, V. D. (2009).
201:
252:
13:
469:10.1016/j.brainresbull.2006.06.012
14:
1993:
1235:Frontiers in Systems Neuroscience
113:
1822:10.1016/j.neuroimage.2014.06.044
1620:10.1016/j.neuroimage.2013.01.049
1520:10.1016/j.neuroimage.2013.07.036
1351:10.1016/j.neuroimage.2009.12.011
1298:10.1016/j.neuroimage.2015.01.057
848:10.1016/j.neuroimage.2010.08.030
423:10.1016/j.neuroimage.2013.05.079
144:
1859:
1644:
1595:
1557:Frontiers in Human Neuroscience
1544:
1476:
1273:
1222:
1155:
1098:
1047:
972:
921:
872:
17:Dynamic functional connectivity
350:Magnetic Resonance in Medicine
168:Independent component analysis
87:The origin of dynamic analysis
1:
760:10.1016/S1053-8119(09)70216-2
725:10.1016/S1053-8119(09)71811-7
282:
96:Significant findings from DFC
1944:10.1371/journal.pone.0039731
1887:10.1016/j.schres.2020.11.055
897:10.1016/j.neulet.2010.11.020
158:anticorrelation between the
60:Positron emission tomography
7:
175:Controversy and limitations
10:
1998:
1485:PLoS Computational Biology
1665:10.1007/s10548-013-0319-5
1060:Frontiers in Neuroscience
799:10.1007/s10334-010-0197-8
1570:10.3389/fnhum.2012.00339
1248:10.3389/fnsys.2013.00101
1073:10.3389/fnins.2016.00381
947:10.3389/fphys.2012.00015
1776:10.1073/pnas.1312902110
1189:10.1073/pnas.1216856110
934:Frontiers in Physiology
584:10.1073/pnas.1018985108
457:Brain Research Bulletin
319:10.1089/brain.2011.0008
150:Time-frequency analysis
103:physiological relevance
51:structural connectivity
34:functional neuroimaging
25:functional connectivity
1875:Schizophrenia Research
362:10.1002/mrm.1910340409
234:Magnetoencephalography
228:
223:Electroencephalography
219:
188:Physiological evidence
132:Point process analysis
83:
213:
164:task-positive network
76:
885:Neuroscience Letters
197:Multi modal approach
160:default mode network
79:default mode network
40:Overview and history
1935:2012PLoSO...739731J
1767:2013PNAS..11018692K
1440:Human Brain Mapping
1388:Human Brain Mapping
1180:2013PNAS..110.4392L
1111:Human Brain Mapping
1007:2014arXiv1405.6466T
985:Human Brain Mapping
575:2011PNAS..108.7641B
504:Human Brain Mapping
277:Alzheimer's disease
240:Neuronal mechanisms
123:Activation patterns
109:Methods of analysis
45:Static connectivity
719:(Suppl. 1): S169.
639:10.1002/jmri.21848
297:Brain Connectivity
262:Clinical relevance
220:
84:
64:resting state fMRI
1452:10.1002/hbm.22140
1446:(12): 3280–3298.
1400:10.1002/hbm.20968
1394:(11): 1713–1726.
1174:(11): 4392–4397.
1123:10.1002/hbm.21513
1015:10.1002/hbm.22562
991:(11): 5442–5456.
754:(Suppl. 1): S57.
559:(18): 7641–7646.
516:10.1002/hbm.20428
202:Electrophysiology
155:wavelet transform
1989:
1967:
1966:
1956:
1946:
1913:
1907:
1906:
1872:
1863:
1857:
1856:
1848:
1842:
1841:
1805:
1799:
1798:
1788:
1778:
1746:
1740:
1737:
1731:
1728:
1722:
1719:
1713:
1710:
1704:
1701:
1695:
1691:
1685:
1684:
1653:Brain Topography
1648:
1642:
1641:
1631:
1599:
1593:
1592:
1582:
1572:
1548:
1542:
1541:
1531:
1499:
1493:
1492:
1480:
1474:
1473:
1463:
1431:
1422:
1421:
1411:
1379:
1373:
1372:
1362:
1326:
1320:
1319:
1309:
1277:
1271:
1270:
1260:
1250:
1226:
1220:
1219:
1209:
1191:
1159:
1153:
1152:
1142:
1117:(6): 1319–1329.
1102:
1096:
1095:
1085:
1075:
1051:
1045:
1044:
1034:
1000:
976:
970:
969:
959:
949:
925:
919:
918:
908:
876:
870:
869:
859:
842:(2): 1140–1150.
827:
821:
820:
810:
778:
772:
771:
743:
737:
736:
708:
702:
701:
691:
682:
676:
675:
667:
661:
660:
650:
618:
607:
606:
596:
586:
568:
544:
538:
537:
527:
495:
489:
488:
463:(4–6): 263–269.
451:
445:
444:
434:
401:
382:
381:
345:
339:
338:
312:
292:
253:Behavioral basis
1997:
1996:
1992:
1991:
1990:
1988:
1987:
1986:
1972:
1971:
1970:
1914:
1910:
1870:
1864:
1860:
1849:
1845:
1806:
1802:
1761:(46): 18692–7.
1747:
1743:
1738:
1734:
1729:
1725:
1720:
1716:
1711:
1707:
1702:
1698:
1692:
1688:
1649:
1645:
1600:
1596:
1549:
1545:
1500:
1496:
1481:
1477:
1432:
1425:
1380:
1376:
1327:
1323:
1278:
1274:
1227:
1223:
1160:
1156:
1103:
1099:
1052:
1048:
977:
973:
926:
922:
877:
873:
828:
824:
779:
775:
744:
740:
709:
705:
689:
683:
679:
668:
664:
619:
610:
545:
541:
496:
492:
452:
448:
402:
385:
346:
342:
310:10.1.1.222.9471
293:
289:
285:
264:
255:
242:
231:
204:
199:
190:
177:
147:
134:
125:
116:
111:
98:
89:
47:
42:
12:
11:
5:
1995:
1985:
1984:
1969:
1968:
1908:
1858:
1843:
1800:
1741:
1732:
1723:
1714:
1705:
1696:
1686:
1643:
1594:
1543:
1494:
1491:(3): e1002438.
1475:
1423:
1374:
1321:
1272:
1221:
1154:
1097:
1046:
971:
920:
891:(2): 158–163.
871:
822:
793:(6): 351–366.
773:
738:
703:
677:
662:
633:(2): 384–393.
608:
539:
510:(6): 671–682.
490:
446:
383:
356:(4): 537–541.
340:
286:
284:
281:
263:
260:
254:
251:
241:
238:
230:
227:
203:
200:
198:
195:
189:
186:
176:
173:
146:
143:
133:
130:
124:
121:
115:
114:Sliding window
112:
110:
107:
97:
94:
88:
85:
46:
43:
41:
38:
9:
6:
4:
3:
2:
1994:
1983:
1980:
1979:
1977:
1964:
1960:
1955:
1950:
1945:
1940:
1936:
1932:
1929:(6): e39731.
1928:
1924:
1920:
1912:
1904:
1900:
1896:
1892:
1888:
1884:
1880:
1876:
1869:
1862:
1854:
1847:
1839:
1835:
1831:
1827:
1823:
1819:
1815:
1811:
1804:
1796:
1792:
1787:
1782:
1777:
1772:
1768:
1764:
1760:
1756:
1752:
1745:
1736:
1727:
1718:
1709:
1700:
1690:
1682:
1678:
1674:
1670:
1666:
1662:
1659:(3): 338–52.
1658:
1654:
1647:
1639:
1635:
1630:
1625:
1621:
1617:
1613:
1609:
1605:
1598:
1590:
1586:
1581:
1576:
1571:
1566:
1562:
1558:
1554:
1547:
1539:
1535:
1530:
1525:
1521:
1517:
1513:
1509:
1505:
1498:
1490:
1486:
1479:
1471:
1467:
1462:
1457:
1453:
1449:
1445:
1441:
1437:
1430:
1428:
1419:
1415:
1410:
1405:
1401:
1397:
1393:
1389:
1385:
1378:
1370:
1366:
1361:
1356:
1352:
1348:
1344:
1340:
1336:
1332:
1331:Glover, G. H.
1325:
1317:
1313:
1308:
1303:
1299:
1295:
1291:
1287:
1283:
1276:
1268:
1264:
1259:
1254:
1249:
1244:
1240:
1236:
1232:
1225:
1217:
1213:
1208:
1203:
1199:
1195:
1190:
1185:
1181:
1177:
1173:
1169:
1165:
1158:
1150:
1146:
1141:
1136:
1132:
1128:
1124:
1120:
1116:
1112:
1108:
1101:
1093:
1089:
1084:
1079:
1074:
1069:
1065:
1061:
1057:
1050:
1042:
1038:
1033:
1028:
1024:
1020:
1016:
1012:
1008:
1004:
999:
994:
990:
986:
982:
975:
967:
963:
958:
953:
948:
943:
939:
935:
931:
924:
916:
912:
907:
902:
898:
894:
890:
886:
882:
875:
867:
863:
858:
853:
849:
845:
841:
837:
833:
826:
818:
814:
809:
804:
800:
796:
792:
788:
784:
777:
769:
765:
761:
757:
753:
749:
742:
734:
730:
726:
722:
718:
714:
707:
699:
695:
688:
681:
673:
666:
658:
654:
649:
644:
640:
636:
632:
628:
624:
617:
615:
613:
604:
600:
595:
590:
585:
580:
576:
572:
567:
562:
558:
554:
550:
543:
535:
531:
526:
521:
517:
513:
509:
505:
501:
494:
486:
482:
478:
474:
470:
466:
462:
458:
450:
442:
438:
433:
428:
424:
420:
416:
412:
408:
400:
398:
396:
394:
392:
390:
388:
379:
375:
371:
367:
363:
359:
355:
351:
344:
336:
332:
328:
324:
320:
316:
311:
306:
302:
298:
291:
287:
280:
278:
274:
273:schizophrenia
270:
259:
250:
246:
237:
235:
226:
224:
217:
212:
208:
194:
185:
181:
172:
169:
165:
161:
156:
151:
145:Other methods
142:
138:
129:
120:
106:
104:
93:
80:
75:
71:
69:
65:
61:
57:
52:
37:
35:
31:
26:
22:
18:
1982:Neuroimaging
1926:
1922:
1911:
1878:
1874:
1861:
1852:
1846:
1813:
1809:
1803:
1758:
1754:
1744:
1735:
1726:
1717:
1708:
1699:
1689:
1656:
1652:
1646:
1611:
1607:
1597:
1560:
1556:
1546:
1511:
1507:
1497:
1488:
1484:
1478:
1443:
1439:
1391:
1387:
1377:
1345:(1): 81–98.
1342:
1338:
1324:
1289:
1285:
1275:
1238:
1234:
1224:
1171:
1167:
1157:
1114:
1110:
1100:
1063:
1059:
1049:
988:
984:
974:
937:
933:
923:
888:
884:
874:
839:
835:
825:
790:
786:
776:
751:
747:
741:
716:
712:
706:
697:
693:
680:
671:
665:
630:
626:
556:
552:
542:
507:
503:
493:
460:
456:
449:
414:
410:
353:
349:
343:
303:(1): 13–36.
300:
296:
290:
265:
256:
247:
243:
232:
221:
205:
191:
182:
178:
148:
139:
135:
126:
117:
99:
90:
48:
20:
16:
15:
1881:: 103–111.
1514:: 826–836.
1329:Chang, C.;
1292:: 476–488.
694:Proc. ISMRM
417:: 360–378.
1816:: 471–80.
1810:NeuroImage
1614:: 227–36.
1608:NeuroImage
1508:NeuroImage
1339:NeuroImage
1286:NeuroImage
836:NeuroImage
748:NeuroImage
713:NeuroImage
411:NeuroImage
283:References
269:depression
1903:231391460
1198:0027-8424
1131:1097-0193
1023:1097-0193
998:1405.6466
566:1010.3775
305:CiteSeerX
68:coherence
1976:Category
1963:22761880
1923:PLOS ONE
1895:33434723
1838:13082197
1830:24973603
1795:24167282
1681:16494240
1673:24104726
1638:23376790
1589:23293596
1538:23876248
1470:22736565
1418:20725910
1369:20006716
1333:(2010).
1316:25662866
1267:24550788
1216:23440216
1149:22331588
1092:27601975
1041:24989126
966:22347863
915:21078369
866:20728554
817:20162320
768:54432053
733:54291742
657:19629982
603:21502525
534:17598166
485:23195652
477:17027761
441:23707587
327:22432952
162:and the
1954:3386248
1931:Bibcode
1786:3832014
1763:Bibcode
1629:3602157
1580:3531919
1563:: 339.
1529:3815981
1461:6870033
1409:6870948
1360:2827259
1307:4386757
1258:3913885
1241:: 101.
1207:3600481
1176:Bibcode
1140:6869909
1083:4994538
1066:: 381.
1032:6869695
1003:Bibcode
957:3274757
906:3014405
857:2997178
808:2891285
700:: 3675.
648:2758521
594:3088578
571:Bibcode
525:6871022
432:3807588
370:8524021
335:6116761
1961:
1951:
1901:
1893:
1836:
1828:
1793:
1783:
1679:
1671:
1636:
1626:
1587:
1577:
1536:
1526:
1468:
1458:
1416:
1406:
1367:
1357:
1314:
1304:
1265:
1255:
1214:
1204:
1196:
1147:
1137:
1129:
1090:
1080:
1039:
1029:
1021:
964:
954:
940:: 15.
913:
903:
864:
854:
815:
805:
766:
731:
655:
645:
601:
591:
532:
522:
483:
475:
439:
429:
378:775793
376:
368:
333:
325:
307:
275:, and
54:using
1899:S2CID
1871:(PDF)
1834:S2CID
1677:S2CID
993:arXiv
764:S2CID
729:S2CID
690:(PDF)
561:arXiv
481:S2CID
374:S2CID
331:S2CID
214:Full
1959:PMID
1891:PMID
1826:PMID
1791:PMID
1669:PMID
1634:PMID
1585:PMID
1534:PMID
1466:PMID
1414:PMID
1365:PMID
1312:PMID
1263:PMID
1212:PMID
1194:ISSN
1145:PMID
1127:ISSN
1088:PMID
1037:PMID
1019:ISSN
962:PMID
911:PMID
862:PMID
813:PMID
653:PMID
599:PMID
530:PMID
473:PMID
437:PMID
366:PMID
323:PMID
77:The
58:and
56:fMRI
30:fMRI
1949:PMC
1939:doi
1883:doi
1879:228
1818:doi
1814:100
1781:PMC
1771:doi
1759:110
1661:doi
1624:PMC
1616:doi
1575:PMC
1565:doi
1524:PMC
1516:doi
1456:PMC
1448:doi
1404:PMC
1396:doi
1355:PMC
1347:doi
1302:PMC
1294:doi
1290:111
1253:PMC
1243:doi
1202:PMC
1184:doi
1172:110
1135:PMC
1119:doi
1078:PMC
1068:doi
1027:PMC
1011:doi
952:PMC
942:doi
901:PMC
893:doi
889:488
852:PMC
844:doi
803:PMC
795:doi
756:doi
721:doi
643:PMC
635:doi
589:PMC
579:doi
557:108
520:PMC
512:doi
465:doi
427:PMC
419:doi
358:doi
315:doi
229:MEG
216:EEG
21:DFC
1978::
1957:.
1947:.
1937:.
1925:.
1921:.
1897:.
1889:.
1877:.
1873:.
1832:.
1824:.
1812:.
1789:.
1779:.
1769:.
1757:.
1753:.
1675:.
1667:.
1657:27
1655:.
1632:.
1622:.
1612:72
1610:.
1606:.
1583:.
1573:.
1559:.
1555:.
1532:.
1522:.
1512:83
1510:.
1506:.
1487:.
1464:.
1454:.
1444:34
1442:.
1438:.
1426:^
1412:.
1402:.
1392:31
1390:.
1386:.
1363:.
1353:.
1343:50
1341:.
1337:.
1310:.
1300:.
1288:.
1284:.
1261:.
1251:.
1237:.
1233:.
1210:.
1200:.
1192:.
1182:.
1170:.
1166:.
1143:.
1133:.
1125:.
1115:34
1113:.
1109:.
1086:.
1076:.
1064:10
1062:.
1058:.
1035:.
1025:.
1017:.
1009:.
1001:.
989:35
987:.
983:.
960:.
950:.
936:.
932:.
909:.
899:.
887:.
883:.
860:.
850:.
840:54
838:.
834:.
811:.
801:.
791:23
789:.
785:.
762:.
752:47
750:.
727:.
717:47
715:.
698:17
696:.
692:.
651:.
641:.
631:30
629:.
625:.
611:^
597:.
587:.
577:.
569:.
555:.
551:.
528:.
518:.
508:29
506:.
502:.
479:.
471:.
461:70
459:.
435:.
425:.
415:80
413:.
409:.
386:^
372:.
364:.
354:34
352:.
329:.
321:.
313:.
299:.
271:,
1965:.
1941::
1933::
1927:7
1905:.
1885::
1855:.
1840:.
1820::
1797:.
1773::
1765::
1683:.
1663::
1640:.
1618::
1591:.
1567::
1561:6
1540:.
1518::
1489:8
1472:.
1450::
1420:.
1398::
1371:.
1349::
1318:.
1296::
1269:.
1245::
1239:7
1218:.
1186::
1178::
1151:.
1121::
1094:.
1070::
1043:.
1013::
1005::
995::
968:.
944::
938:3
917:.
895::
868:.
846::
819:.
797::
770:.
758::
735:.
723::
659:.
637::
605:.
581::
573::
563::
536:.
514::
487:.
467::
443:.
421::
380:.
360::
337:.
317::
301:1
19:(
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.