68:
518:
178:
471:. Some patients with polymicrogyria go undiagnosed until they produce children with the disorder who have more severe manifestations. Retrospectively, these patients will often report some difficulty in their medical or educational history. BFPP patients demonstrate mental retardation, language impairment, motor developmental delay, and seizure disorders such as epilepsy. The association of epilepsy is in approximately 50% to 85% of affected BFPP patients.
615:
or adulthood, those with mild forms may have no obvious deficit or only minor manifestations, such as a simple lisp or isolated learning disability. Therefore, if a familial polymicrogyria syndrome is suspected, it may be reasonable to perform MRI on relatives who are asymptomatic or have what appear to be minor findings. The presence of consanguinity in a child's parents may suggest an autosomal recessive familial polymicrogyria syndrome.
140:
125:. In lissencephaly, few or no sulci are seen on the cortical surface, resulting in a broad, smooth appearance to the entire brain. Lissencephaly can be radiologically confused with polymicrogyria, particularly with low-resolution imaging, but the smoothness and lack of irregularity in the gray-white junction, along with markedly increased cortical thickness, distinguishes lissencephaly.
584:. Treatment measures may include physical therapy, occupational therapy, Speech therapy, anti-seizure drugs and orthotic devices. Surgery may be needed to assuage spastic motor problems. Various supportive measures such as joint contractures that could prevent complications. Genetic counseling may also be recommended
110:, and cerebellar dysplasia, usually distinguishes these disorders from polymicrogyria. There are no anatomopathologic studies that have characterized the pattern of cortical laminar alterations in patients with GPR56 gene mutations, but it has been suggested that the imaging characteristics of BFPP, including
614:
signs, and focal weakness because many affected family members, particularly those who are older, may not have had MRI performed, even if these problems came to medical attention. In addition, although most individuals with polymicrogyria do present with neurologic difficulties in infancy, childhood,
494:
Individuals with the milder forms of polymicrogyria survive into adulthood, while those with the most severe forms, such as BFPP, may die at a young age as a result of such complications as seizures or pneumonia. The prevalence of isolated polymicrogyria is unknown. Researchers believe that it may be
521:
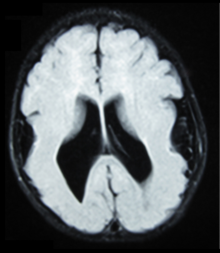
This child presented with seizures. The coronal true inversion recovery sequence shows thickened and disordered cortex in superior frontal and cingulate gyri bilaterally (arrow). There are small convolutions visible at the corticomedullary junction. The appearance is that of cortical dysplasia, with
53:
and patients with polymicrogyria have an increase number of folds and smaller folds than usual. Polymicrogyria is defined as a cerebral malformation of cortical development in which the normal gyral pattern of the surface of the brain is replaced by an excessive number of small, fused gyri separated
567:
Microscopic examination demonstrates that the cerebral cortex is in fact abnormally thin and has abnormal lamination; typically the cortex is unlayered or has four layers, in contrast to the normal six layers. The most superficial layers between adjacent small gyri appear fused, with the pia (layer
534:, Behavioral assay, Electron microscopy, CT scan, or MRI demonstrate different results that concludes an affected BFPP patient. MRI's reveal either irregularity to the cortical surface suggestive of multiple small folds or an irregular, scalloped appearance of the gray matter-white matter junction.
439:
genotype for a deletion of chromosome 16q12.1-q21 region, including GPR56 gene. To date the only gene known to be associated with polymicrogyria is GPR56. Testing for GPR56-related bilateral frontoparietal polymicrogyria is available clinically. Mutations in GPR56 hinders
Collagen III, its specific
135:
Polymicrogyria is often confused with pachygyria; therefore, it needs to be distinguished from pachygyria, a distinct brain malformation in which the surface folds are excessively broad and sparse. Pachygyria and polymicrogyria may look similar on low-resolution neuroimaging such as CT because the
92:
BFPP is a cobblestone-like cortical malformation of the brain. Disruptions of cerebral cortical development due to abnormal neuronal migration and positioning usually lead to cortical disorders, which includes cobblestone lissencephaly. Cobblestone lissencephaly is typically seen in three different
58:
and abnormal cortical lamination. From ongoing research, mutation in GPR56, a member of the adhesion G protein-coupled receptor (GPCR) family, results in BFPP. These mutations are located in different regions of the protein without any evidence of a relationship between the position of the mutation
105:
that have migrated beyond the normal surface boundaries of the brain. Sometimes regions populated by these misplaced cells have caused a radiologic misdiagnosis of polymicrogyria. However, the presence of other abnormalities in these cobblestone lissencephaly syndromes, including ocular anomalies,
169:
arranged in a specific fashion (C-x2-W-x6-16-W-x4-C-x10-22-C-x-C) just before the first transmembrane domain and serves as a cleavage site in some members of this group of G protein–coupled receptors. Although, the molecular and cellular mechanisms of how GPR56 regulates brain development remain
132:. This illness can be cryptogenic or symptomatic, but the symptomatic forms have been associated with multiple etiologies and abnormal cortical development. BFPP caused by GPR56 mutations is a manifestation of a malformation of cortical development that causes Lennox-Gastaut Syndrome.
346:
In populations with a high rate of consanguinity, the offspring of a person with GPR56-related BFPP and a reproductive partner who is a carrier of GPR56-related BFPP have a 50% chance of inheriting two GPR56 disease-causing alleles and having BFPP and a 50% chance of being
522:
polymicrogyria more likely than pachygyria due to the small convolutions visible. There are also small foci of grey matter signal in the corpus callosum, deep to the dysplastic cortex (double arrows). These probably represent areas of grey matter heterotopia.
631:
malformations that could indicate a particular syndrome. Neurologic examination should assess cognitive and mental abilities, cranial nerve function, motor function, deep tendon reflexes, sensory function, coordination, and gait (if appropriate).
143:
Lissencephaly:Brain MRI, T1 weighted, transverse plane, that shows lyssencephaly, manifested as scarce and wide circumvolutions, mostly in the occipital, parietal and temporal lobes. As aggregated findings, there is ventriculomegaly, no true
563:
to the cerebral cortex, with miniature gyri fused and superimposed together, often resulting in an irregular brain surface. The cortical ribbon can appear excessively thick as a result of the infolding and fusion of multiple small gyri.
1005:
Borgatti R, Marelli S, Bernardini L, Novelli A, Cavallini A, Tonelli A, et al. (December 2009). "Bilateral frontoparietal polymicrogyria (BFPP) syndrome secondary to a 16q12.1-q21 chromosome deletion involving GPR56 gene".
683:
Bahi-Buisson N, Poirier K, Boddaert N, Fallet-Bianco C, Specchio N, Bertini E, et al. (November 2010). "GPR56-related bilateral frontoparietal polymicrogyria: further evidence for an overlap with the cobblestone complex".
550:
or adequate contrast to identify the small folds that define the condition. The cerebral cortex often appears abnormally thick as well because the multiple small gyri are fused, infolded, and superimposed in appearance.
118:, are reminiscent of those of the so-called cobblestone malformations (muscle-eye-brain disease and Fukuyama congenital muscular dystrophy) that are also associated with N-glycosylation defects in the developing brain.
136:
cortical thickness can appear to be increased and the gyri can appear to be broad and smooth in both conditions. This is why higher resolution neuroimaging, such as an MRI, is necessary for proper diagnosis.
580:
Treatment plans will vary depending on the severity of the condition and its evidences in each patient. Areas that will probably need to be evaluated and assessed include speech, vision, hearing and
599:
infections associated with polymicrogyria with standard TORCH testing may be appropriate. Other specific tests targeting individual neurometabolic disorders can be obtained if clinically suggested.
279:
At conception, each sibling of an affected individual has a 25% chance of being affected, a 50% chance of being an asymptomatic carrier, and a 25% chance of being unaffected and not a carrier.
780:
Stutterd CA, Dobyns WB, Jansen A, Mirzaa G, Leventer RJ (1993). "Polymicrogyria
Overview". In Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, Gripp KW, Amemiya A (eds.).
101:, and muscle-eye-brain disease. In cobblestone lissencephaly, the brain surface actually has a bumpy contour caused by the presence of collections of misplaced neurons and
170:
largely unknown. These types of receptors play an essential role in biological processes including embryonic development, central nervous system (CNS), immune system, and
860:
Piao X, Chang BS, Bodell A, Woods K, Benzeev B, Topcu M, et al. (November 2005). "Genotype-phenotype analysis of human frontoparietal polymicrogyria syndromes".
499:
Radiological findings (MRI) demonstrated symmetric generalized polymicrogyria with decreasing anterior-posterior gradient, most prominent in frontoparietal cortex.
595:
A pregnancy history should be sought, with particular regard to infections, trauma, multiple gestations, and other documented problems. Screening for the common
157:
The GPR56 is grouped in the B family of GPCRs. This GPCR group have long N termini characterized by an extracellular “cysteine box” and hydrophilic, potentially
1225:
Guerrini R, Dobyns WB, Barkovich AJ (March 2008). "Abnormal development of the human cerebral cortex: genetics, functional consequences and treatment options".
444:, to bind in a developing brain. To date, a total of fourteen BFPP-associated mutations have been identified, including one deletion, two splicing, and eleven
467:
compartment via interaction with this type of receptor. Children often present with developmental delay, spasticity, or seizures; they are also often
448:. Two mutations in the GPCR proteolytic site (GPS) domain, C346S and W349S, cause a brain malformation through trapping the mutated proteins in the
484:
In more severe forms, focal motor, sensory, visual, or cognitive problems may be present, depending on the location of the brain region affected.
546:
is typically made by magnetic resonance imaging (MRI) since computed tomography (CT) and other imaging methods generally do not have high enough
592:
Once the diagnosis of polymicrogyria has been established in an individual, the following approach can be used for discussion of prognosis:
530:
expression or visuals of the brain to analyze the specific sections that are affected. These tests for example, using animals such as mice,
610:
It is important to ask for the presence of neurologic problems in family members, including seizures, cognitive delay, motor impairment,
572:
diagnosis for BFPP is also available for pregnancies at risk if the GPR56 mutations have been identified in an affected family member.
1367:
94:
669:
1279:
897:"G protein-coupled receptor 56 and collagen III, a receptor-ligand pair, regulates cortical development and lamination"
648:
1348:
128:
GPR56 mutation also can cause a severe encephalopathy which is associated with electro clinical features of
1330:
956:"Disease-associated GPR56 mutations cause bilateral frontoparietal polymicrogyria via multiple mechanisms"
463:. Within this family there are different types of bio-active molecules that transduce their signal to the
1111:"Characterization of G protein-coupled receptor 56 protein expression in the mouse developing neocortex"
491:
and generalized, resulting in severe intellectual disability, cerebral palsy, and refractory epilepsy.
1290:
129:
98:
282:
Once an at-risk sibling is known to be unaffected, the risk of his/her being a carrier is 2/3.
449:
343:
Offspring of a proband are obligate heterozygotes and will therefore carry one mutant allele.
55:
813:"Bilateral frontoparietal polymicrogyria, Lennox-Gastaut syndrome, and GPR56 gene mutations"
83:
delay, mental retardation - moderate to severe, exaggerated reflexes and seizures (epilepsy)
1344:
908:
481:
with only one small region of the brain involved; neurologic problems may not be evident.
80:
8:
218:
The parents of an affected individual are obligate heterozygotes and therefore carry one
59:
and phenotypic severity. It is also found that GPR56 plays a role in cortical pattering.
42:
912:
1202:
1178:"GPR56-regulated granule cell adhesion is essential for rostral cerebellar development"
1177:
1135:
1110:
1067:
1042:
982:
955:
931:
896:
837:
812:
732:
719:
Piao X, Walsh CA (September 2004). "A novel signaling mechanism in brain development".
954:
Chiang NY, Hsiao CC, Huang YS, Chen HY, Hsieh IJ, Chang GW, et al. (April 2011).
1301:
1242:
1207:
1140:
1072:
1023:
1019:
987:
936:
877:
842:
828:
787:
736:
701:
445:
1234:
1197:
1193:
1189:
1130:
1122:
1062:
1054:
1015:
977:
967:
926:
916:
869:
832:
824:
728:
693:
547:
107:
1158:
1091:
1295:
1043:"GPR56 and the developing cerebral cortex: cells, matrix, and neuronal migration"
636:
145:
46:
1306:
1238:
901:
Proceedings of the
National Academy of Sciences of the United States of America
543:
1058:
488:
474:
The clinical manifestations of polymicrogyria are stable neurologic deficits:
1361:
781:
468:
464:
171:
67:
972:
921:
45:
inheritance that causes a cortical malformation. Our brain has folds in the
1352:
1246:
1211:
1144:
1076:
1027:
991:
940:
881:
846:
791:
740:
705:
697:
624:
611:
436:
286:
560:
404:
Each sibling of the proband's parents is at a 50% risk of being a carrier
111:
1271:
682:
517:
596:
478:
166:
122:
102:
1126:
873:
623:
A general physical examination of the proband may identify associated
177:
115:
148:, too thick gray matter and ectopic gray matter in the white matter.
1325:
628:
569:
162:
811:
Parrini E, Ferrari AR, Dorn T, Walsh CA, Guerrini R (June 2009).
375:
1284:
1004:
139:
895:
Luo R, Jeong SJ, Jin Z, Strokes N, Li S, Piao X (August 2011).
559:
Gross neuropathologic examination reveals a pattern of complex
460:
441:
219:
527:
158:
50:
1109:
Jeong SJ, Luo R, Li S, Strokes N, Piao X (September 2012).
531:
495:
relatively common overall, although BFPP is probably rare.
456:
779:
602:
The following may help in determining a genetic etiology:
459:
family, the largest cell surface gene family in the human
581:
753:
Lin, Dr. Hsi-Hsien. Personal
Interview. 29 October 2012.
1224:
810:
526:
There are different tests or methods used to determine
121:
Lissencephaly ("smooth brain") is the extreme form of
1261:
1175:
953:
859:
373:
71:
Left:Normal Middle:polymicrgyria Right:Lissencephaly
1041:Singer K, Luo R, Jeong SJ, Piao X (February 2013).
1040:
894:
1108:
435:Diagnostic criteria for a BFPP patient entails a
1359:
1176:Koirala S, Jin Z, Piao X, Corfas G (June 2009).
670:"Bilateral Frontoparietal Polymicrogyria (BFPP)"
568:of the meninges) bridging across multiple gyri.
161:-rich. The cysteine box contains four conserved
93:human congenital muscular dystrophy syndromes:
796:Retired Chapter, for Historical Reference Only
487:In the most severe forms, polymicrogyria is
806:
804:
225:Heterozygotes (carriers) are asymptomatic.
1201:
1134:
1066:
981:
971:
930:
920:
836:
718:
315:
1345:Bilateral Frontalparietal Polymicrogyria
667:
516:
176:
138:
87:
66:
24:Bilateral frontalparietal polymicrogyria
801:
775:
773:
771:
769:
767:
765:
763:
761:
759:
618:
477:In the mildest form, polymicrogyria is
455:GPR56 are a part of the B class of the
190:
39:Bilateral frontoparietal polymicrogyria
1360:
185:
95:Fukuyama congenital muscular dystrophy
786:. University of Washington, Seattle.
1165:. U.S. National Library of Medicine.
1115:The Journal of Comparative Neurology
756:
251:
960:The Journal of Biological Chemistry
13:
1089:
733:10.1203/01.PDR.0000139720.67707.D0
635:
14:
1379:
1257:
605:
554:
1020:10.1111/j.1399-0004.2009.01262.x
829:10.1111/j.1528-1167.2008.01787.x
512:
114:defects and cerebellar cortical
49:to increase surface area called
1218:
1169:
1151:
1102:
1083:
1034:
998:
649:Epilepsy Phenome/Genome Project
537:
106:congenital muscular dystrophy,
62:
1368:Genetic diseases and disorders
1194:10.1523/JNEUROSCI.1182-09.2009
947:
888:
853:
747:
712:
676:
668:Guerrini R (5 November 2012).
661:
1:
654:
285:Heterozygotes (carriers) are
587:
575:
502:Numerous gyrus on the cortex
430:
7:
1182:The Journal of Neuroscience
642:
152:
41:is a genetic disorder with
10:
1384:
1239:10.1016/j.tins.2007.12.004
374:Other family members of a
1316:
1265:
1059:10.1007/s12035-012-8343-0
28:
23:
1227:Trends in Neurosciences
1163:Genetics Home Reference
973:10.1074/jbc.M110.183830
922:10.1073/pnas.1104821108
130:Lennox-Gastaut syndrome
99:Walker-Warburg syndrome
79:: developmental delay,
1047:Molecular Neurobiology
627:, musculoskeletal, or
523:
316:Offspring of a proband
182:
149:
72:
520:
450:endoplasmic reticulum
180:
142:
88:Associated conditions
70:
698:10.1093/brain/awq259
619:Physical examination
505:Small gyri and sulci
191:Parents of a proband
966:(16): 14215–14225.
913:2011PNAS..10812925L
907:(31): 12925–12930.
862:Annals of Neurology
186:Mode of inheritance
181:GPCR classification
43:autosomal recessive
1317:External resources
721:Pediatric Research
524:
446:missense mutations
183:
150:
73:
1340:
1339:
1188:(23): 7439–7449.
1127:10.1002/cne.23076
1121:(13): 2930–2940.
1008:Clinical Genetics
874:10.1002/ana.20616
692:(11): 3194–3209.
542:The diagnosis of
252:Sibs of a proband
36:
35:
18:Medical condition
1375:
1263:
1262:
1251:
1250:
1222:
1216:
1215:
1205:
1173:
1167:
1166:
1159:"Polymicrogyria"
1155:
1149:
1148:
1138:
1106:
1100:
1099:
1092:"Polymicrogyria"
1087:
1081:
1080:
1070:
1038:
1032:
1031:
1002:
996:
995:
985:
975:
951:
945:
944:
934:
924:
892:
886:
885:
857:
851:
850:
840:
823:(6): 1344–1353.
808:
799:
798:
777:
754:
751:
745:
744:
716:
710:
709:
680:
674:
673:
665:
108:ventriculomegaly
21:
20:
1383:
1382:
1378:
1377:
1376:
1374:
1373:
1372:
1358:
1357:
1341:
1336:
1335:
1312:
1311:
1274:
1260:
1255:
1254:
1223:
1219:
1174:
1170:
1157:
1156:
1152:
1107:
1103:
1088:
1084:
1039:
1035:
1003:
999:
952:
948:
893:
889:
858:
854:
809:
802:
778:
757:
752:
748:
717:
713:
681:
677:
666:
662:
657:
645:
640:
637:Genetic testing
621:
608:
590:
578:
557:
540:
515:
433:
379:
318:
254:
193:
188:
155:
146:Sylvian fissure
90:
65:
19:
12:
11:
5:
1381:
1371:
1370:
1356:
1355:
1338:
1337:
1334:
1333:
1321:
1320:
1318:
1314:
1313:
1310:
1309:
1298:
1287:
1275:
1270:
1269:
1267:
1266:Classification
1259:
1258:External links
1256:
1253:
1252:
1233:(3): 154–162.
1217:
1168:
1150:
1101:
1082:
1053:(1): 186–196.
1033:
1014:(6): 573–576.
997:
946:
887:
868:(5): 680–687.
852:
800:
755:
746:
727:(3): 309–310.
711:
675:
659:
658:
656:
653:
652:
651:
644:
641:
639:
634:
620:
617:
607:
606:Family history
604:
589:
586:
577:
574:
556:
555:Neuropathology
553:
544:polymicrogyria
539:
536:
514:
511:
510:
509:
506:
503:
500:
432:
429:
428:
427:
426:
425:
424:
423:
422:
421:
420:
419:
418:
417:
416:
415:
414:
413:
412:
411:
410:
409:
408:
407:
406:
405:
378:
372:
371:
370:
369:
368:
367:
366:
365:
364:
363:
362:
361:
360:
359:
358:
357:
356:
355:
354:
353:
352:
351:
350:
349:
348:
344:
317:
314:
313:
312:
311:
310:
309:
308:
307:
306:
305:
304:
303:
302:
301:
300:
299:
298:
297:
296:
295:
294:
293:
292:
291:
290:
283:
280:
253:
250:
249:
248:
247:
246:
245:
244:
243:
242:
241:
240:
239:
238:
237:
236:
235:
234:
233:
232:
231:
230:
229:
228:
227:
226:
223:
192:
189:
187:
184:
154:
151:
89:
86:
85:
84:
64:
61:
34:
33:
30:
26:
25:
17:
9:
6:
4:
3:
2:
1380:
1369:
1366:
1365:
1363:
1354:
1353:Rare Diseases
1351:'s Office of
1350:
1346:
1343:
1342:
1332:
1328:
1327:
1323:
1322:
1319:
1315:
1308:
1304:
1303:
1299:
1297:
1293:
1292:
1288:
1286:
1282:
1281:
1277:
1276:
1273:
1268:
1264:
1248:
1244:
1240:
1236:
1232:
1228:
1221:
1213:
1209:
1204:
1199:
1195:
1191:
1187:
1183:
1179:
1172:
1164:
1160:
1154:
1146:
1142:
1137:
1132:
1128:
1124:
1120:
1116:
1112:
1105:
1097:
1093:
1086:
1078:
1074:
1069:
1064:
1060:
1056:
1052:
1048:
1044:
1037:
1029:
1025:
1021:
1017:
1013:
1009:
1001:
993:
989:
984:
979:
974:
969:
965:
961:
957:
950:
942:
938:
933:
928:
923:
918:
914:
910:
906:
902:
898:
891:
883:
879:
875:
871:
867:
863:
856:
848:
844:
839:
834:
830:
826:
822:
818:
814:
807:
805:
797:
793:
789:
785:
784:
776:
774:
772:
770:
768:
766:
764:
762:
760:
750:
742:
738:
734:
730:
726:
722:
715:
707:
703:
699:
695:
691:
687:
679:
671:
664:
660:
650:
647:
646:
638:
633:
630:
626:
616:
613:
603:
600:
598:
593:
585:
583:
573:
571:
565:
562:
552:
549:
545:
535:
533:
529:
519:
513:Methods/tests
507:
504:
501:
498:
497:
496:
492:
490:
485:
482:
480:
475:
472:
470:
469:microcephalic
466:
465:intracellular
462:
458:
453:
451:
447:
443:
438:
403:
402:
401:
400:
399:
398:
397:
396:
395:
394:
393:
392:
391:
390:
389:
388:
387:
386:
385:
384:
383:
382:
381:
380:
377:
345:
342:
341:
340:
339:
338:
337:
336:
335:
334:
333:
332:
331:
330:
329:
328:
327:
326:
325:
324:
323:
322:
321:
320:
319:
288:
284:
281:
278:
277:
276:
275:
274:
273:
272:
271:
270:
269:
268:
267:
266:
265:
264:
263:
262:
261:
260:
259:
258:
257:
256:
255:
224:
221:
217:
216:
215:
214:
213:
212:
211:
210:
209:
208:
207:
206:
205:
204:
203:
202:
201:
200:
199:
198:
197:
196:
195:
194:
179:
175:
173:
172:tumorigenesis
168:
164:
160:
147:
141:
137:
133:
131:
126:
124:
119:
117:
113:
109:
104:
100:
96:
82:
78:
75:
74:
69:
60:
57:
52:
48:
44:
40:
31:
27:
22:
16:
1324:
1300:
1289:
1278:
1230:
1226:
1220:
1185:
1181:
1171:
1162:
1153:
1118:
1114:
1104:
1095:
1085:
1050:
1046:
1036:
1011:
1007:
1000:
963:
959:
949:
904:
900:
890:
865:
861:
855:
820:
816:
795:
782:
749:
724:
720:
714:
689:
685:
678:
663:
625:craniofacial
622:
612:pseudobulbar
609:
601:
594:
591:
579:
566:
561:convolutions
558:
541:
538:Neuroimaging
525:
493:
486:
483:
476:
473:
454:
437:heterozygous
434:
287:asymptomatic
156:
134:
127:
120:
91:
76:
63:Presentation
38:
37:
15:
1090:Sarnat HB.
783:GeneReviews
508:Thin cortex
167:tryptophans
112:myelination
103:glial cells
81:psychomotor
54:by shallow
29:Other names
1302:DiseasesDB
655:References
597:congenital
548:resolution
479:unilateral
123:pachygyria
817:Epilepsia
588:Prognosis
576:Treatment
489:bilateral
431:Diagnosis
347:carriers.
163:cysteines
116:dysplasia
1362:Category
1326:Orphanet
1247:18262290
1212:19515912
1145:22351047
1077:23001883
1028:19807741
992:21349848
941:21768377
882:16240336
847:19016831
792:20301504
741:15269343
706:20929962
643:See also
629:visceral
570:Prenatal
165:and two
153:Genetics
77:Symptoms
1296:C564652
1203:2744696
1136:3908671
1096:MedLink
1068:3538897
983:3077623
932:3150909
909:Bibcode
838:4271835
376:proband
222:allele.
1331:101070
1285:606854
1245:
1210:
1200:
1143:
1133:
1075:
1065:
1026:
990:
980:
939:
929:
880:
845:
835:
790:
739:
704:
461:genome
442:ligand
220:mutant
47:cortex
1307:33974
686:Brain
528:GPR56
159:mucin
56:sulci
1291:MeSH
1280:OMIM
1243:PMID
1208:PMID
1141:PMID
1073:PMID
1024:PMID
988:PMID
937:PMID
878:PMID
843:PMID
788:PMID
737:PMID
702:PMID
532:RNAi
457:GPCR
51:gyri
32:BFPP
1349:NIH
1347:at
1235:doi
1198:PMC
1190:doi
1131:PMC
1123:doi
1119:520
1063:PMC
1055:doi
1016:doi
978:PMC
968:doi
964:286
927:PMC
917:doi
905:108
870:doi
833:PMC
825:doi
729:doi
694:doi
690:133
582:EEG
1364::
1329::
1305::
1294::
1283::
1241:.
1231:31
1229:.
1206:.
1196:.
1186:29
1184:.
1180:.
1161:.
1139:.
1129:.
1117:.
1113:.
1094:.
1071:.
1061:.
1051:47
1049:.
1045:.
1022:.
1012:76
1010:.
986:.
976:.
962:.
958:.
935:.
925:.
915:.
903:.
899:.
876:.
866:58
864:.
841:.
831:.
821:50
819:.
815:.
803:^
794:.
758:^
735:.
725:56
723:.
700:.
688:.
452:.
174:.
97:,
1272:D
1249:.
1237::
1214:.
1192::
1147:.
1125::
1098:.
1079:.
1057::
1030:.
1018::
994:.
970::
943:.
919::
911::
884:.
872::
849:.
827::
743:.
731::
708:.
696::
672:.
289:.
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.