1345:
1313:
2240:
978:
46:
2251:
1259:
1054:
1232:
1373:
1180:
1850:
Smith, Amos B.; Zhu, Wenyu; Shirakami, Shohei; Sfouggatakis, Chris; Doughty, Victoria A.; Bennett, Clay S.; Sakamoto, Yasuharu (2003-03-01). "Total
Synthesis of (+)-Spongistatin 1. An Effective Second-Generation Construction of an Advanced EF Wittig Salt, Fragment Union, and Final Elaboration".
2164:
Zhou, Hao; Liao, Xuebin; Cook, James M. (2004-01-01). "Regiospecific, Enantiospecific Total
Synthesis of the 12-Alkoxy-Substituted Indole Alkaloids, (+)-12-Methoxy-Na-methylvellosimine, (+)-12-Methoxyaffinisine, and (−)-Fuchsiaefoline".
1477:
Zhang, Xian-Man; Bordwell, Frederick G. (1992). "Homolytic bond dissociation energies of the benzylic carbon-hydrogen bonds in radical anions and radical cations derived from fluorenes, triphenylmethanes, and related compounds".
1921:
Takaku, Hiroshi; Kamaike, Kazuo; Tsuchiya, Hiromichi (1984-01-01). "Oligonucleotide synthesis. Part 21. Synthesis of ribooligonucleotides using the 4-methoxybenzyl group as a new protecting group for the 2′-hydroxyl group".
936:
Benzyl groups are occasionally employed as protecting groups in organic synthesis. Their installation and especially their removal require relatively harsh conditions, so benzyl is not typically preferred for protection.
850:
360:
is about 10–15% weaker than other kinds of C−H bonds. The neighboring aromatic ring stabilizes benzyl radicals. The data tabulated below compare benzylic C−H bond to related C−H bond strengths.
2137:
Cain, Christian M.; Cousins, Richard P. C.; Coumbarides, Greg; Simpkins, Nigel S. (1990-01-01). "Asymmetric deprotonation of prochiral ketones using chiral lithium amide bases".
2014:
Mukaiyama, Teruaki; Shiina, Isamu; Iwadare, Hayato; Saitoh, Masahiro; Nishimura, Toshihiro; Ohkawa, Naoto; Sakoh, Hiroki; Nishimura, Koji; Tani, Yu-ichirou (1999-01-04).
2055:
Hanessian, Stephen; Marcotte, Stéphane; Machaalani, Roger; Huang, Guobin (2003-11-01). "Total
Synthesis and Structural Confirmation of Malayamycin A: A Novel Bicyclic
2208:
Rong, Yi; Al-Harbi, Ahmed; Parkin, Gerard (2012). "Highly
Variable Zr–CH2–Ph Bond Angles in Tetrabenzylzirconium: Analysis of Benzyl Ligand Coordination Modes".
288:. None of these species can be formed in significant amounts in the solution phase under normal conditions, but they are useful referents for discussion of
653:
The weakness of the C−H bond reflects the stability of the benzylic radical. For related reasons, benzylic substituents exhibit enhanced reactivity, as in
304:
can be represented as BnOH. Less common abbreviations are Bzl and Bz, the latter of which is ambiguous as it is also the standard abbreviation for the
1507:
1624:
1577:
2102:
Kuehne, Martin E.; Xu, Feng (1993-12-01). "Total synthesis of strychnan and aspidospermatan alkaloids. 3. The total synthesis of (±)-strychnine".
1573:
1761:
Fukuzawa, Akio; Sato, Hideaki; Masamune, Tadashi (1987-01-01). "Synthesis of (±)-prepinnaterpene, a bromoditerpene from the red alga Yamada".
1442:
Xue, Xiao-Song; Ji, Pengju; Zhou, Biying; Cheng, Jin-Pei (2017). "The
Essential Role of Bond Energetics in C–H Activation/Functionalization".
2283:
2032:
2015:
1788:
Van Hijfte, Luc; Little, R. Daniel (1985-10-01). "Intramolecular 1,3-diyl trapping reactions. A formal total synthesis of (±)-coriolin".
683:
3202:
1894:
Marco, José L.; Hueso-Rodríguez, Juan A. (1988-01-01). "Synthesis of optically pure 1-(3-furyl)-1,2-dihydroxyethane derivatives".
3207:
1210:
1823:
Sirkecioglu, Okan; Karliga, Bekir; Talinli, Naciye (2003-11-10). "Benzylation of alcohols by using biscopper as catalyst".
1600:
1524:
2255:
1737:
1655:
1549:
1426:
1670:
Baran, Phil S.; Zhong, Yong-Li (2001-04-01). "Selective
Oxidation at Carbon Adjacent to Aromatic Systems with IBX".
878:). Any non-tertiary benzylic alkyl group will be oxidized to a carboxyl group by aqueous potassium permanganate (
2276:
3106:
1220:
864:
3235:
2679:
2269:
863:
In a few cases, these benzylic transformations occur under conditions suitable for lab synthesis. The
658:
1595:. Coxon, J. M. (James Morriss), 1941- (3rd. ed.). London: Blackie Academic & Professional.
2716:
1418:
1268:
Conditions for deprotection of benzyl group are applicable for cleavage of the PMB protecting group
341:
3189:
1330:
1207:
4-methoxybenzyl-2,2,2-trichloroacetimidate can be used to install the PMB group in presence of:
3089:
1010:
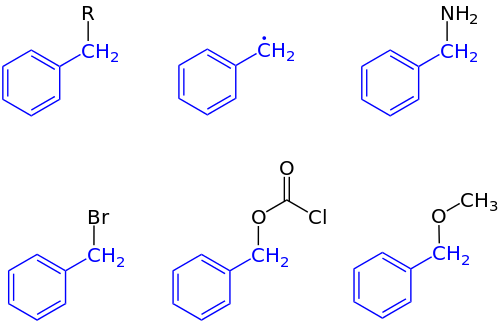
17:
3196:
3084:
1410:
3230:
3165:
2610:
1377:
1173:
925:
905:
598:
65:
1086:
Benzyl protecting groups can be removed using a wide range of oxidizing agents including:
8:
2471:
1411:
1294:
1193:
958:
946:
53:
1991:
1958:
1743:
1618:
1567:
1505:
Sheehan, Richard J. "Terephthalic Acid, Dimethyl
Terephthalate, and Isophthalic Acid".
1107:
289:
147:
2150:
1907:
1774:
3155:
3125:
2883:
2505:
2190:
2182:
2119:
2084:
2076:
2037:
1996:
1978:
1939:
1876:
1868:
1805:
1733:
1695:
1687:
1651:
1606:
1596:
1555:
1545:
1520:
1459:
1422:
1326:
1286:
1169:
1116:
1089:
1000:
901:
674:
85:
1747:
2860:
2354:
2292:
2244:
2217:
2174:
2146:
2111:
2068:
2027:
1986:
1970:
1931:
1903:
1860:
1832:
1797:
1770:
1725:
1679:
1643:
1512:
1487:
1451:
1278:
1161:
1344:
196:
is used to describe the position of the first carbon bonded to a benzene or other
3079:
2838:
2833:
2816:
2799:
2600:
2349:
1836:
1298:
1224:
1137:
1006:
992:
966:
950:
174:
170:
127:
1455:
3150:
3145:
3021:
3016:
3011:
2804:
2771:
2555:
2537:
2527:
1302:
1197:
1129:
1047:
970:
962:
662:
654:
301:
61:
1559:
1140:
at ambient temperature (selectivity can be achieved under specific conditions)
945:
Benzyl is commonly used in organic synthesis as a robust protecting group for
855:
Millions of tonnes of terephthalic acid are produced annually by this method.
3224:
3170:
3118:
3049:
2935:
2925:
2920:
2910:
2905:
2855:
2850:
2766:
2761:
2751:
2605:
2560:
2522:
2510:
2481:
2359:
2186:
2123:
2080:
2041:
1982:
1943:
1872:
1809:
1691:
1647:
1516:
1361:
2033:
10.1002/(SICI)1521-3765(19990104)5:1<121::AID-CHEM121>3.0.CO;2-O
1610:
1009:
can be selectively benzylated in presence of phenol functional groups using
3101:
2988:
2983:
2960:
2711:
2550:
2476:
2408:
2386:
2342:
2327:
2317:
2194:
2088:
2000:
1880:
1699:
1463:
1322:
221:
57:
1312:
3160:
3113:
3074:
2955:
2843:
2828:
2823:
2811:
2376:
2371:
2337:
2332:
2322:
2300:
1390:
1096:
460:
241:
93:
2115:
1935:
1801:
1638:
Johnston, Jeffrey N. (2001), "Chromium(VI) Oxide–3,5-Dimethylpyrazole",
1491:
3069:
3060:
2940:
2895:
2791:
2756:
2746:
2686:
2622:
2545:
2493:
1729:
340:
The enhanced reactivity of benzylic positions is attributed to the low
2221:
2178:
2072:
1974:
1864:
1683:
916:) will selectively oxidize a benzylic methylene group to a carbonyl: (
3036:
2950:
2915:
2900:
2888:
2731:
2706:
2515:
1365:
1033:
977:
561:
265:
2261:
845:{\displaystyle {\ce {CH3C6H4CH3 + 3 O2 -> HO2CC6H4CO2H + 2 H2O}}}
3044:
2998:
2965:
2661:
2567:
2441:
2396:
2381:
1338:
1306:
1165:
197:
45:
1258:
3006:
2930:
2781:
2776:
2741:
2726:
2721:
2691:
2674:
2498:
2425:
2391:
2250:
1075:
1068:
1053:
665:. As a practical example, in the presence of suitable catalysts,
527:
305:
112:
35:
2054:
320:. Likewise, benzyl should not be confused with the phenyl group
3094:
3026:
2870:
2579:
2572:
2466:
2447:
2436:
2420:
2366:
1064:
670:
635:
497:
430:
178:
39:
31:
2136:
2975:
2945:
2878:
2736:
2701:
2696:
2669:
2617:
2584:
2488:
2312:
1849:
1282:
1231:
1102:
1026:
858:
77:
69:
2013:
1413:
Advanced
Organic Chemistry, Part A: Structure and Mechanisms
1179:
2403:
1957:
Trost, Barry M.; Waser, Jerome; Meyer, Arndt (2007-11-01).
988:
73:
1822:
1372:
957:
Treatment of alcohol with a strong base such as powdered
835:
812:
799:
786:
773:
757:
737:
724:
711:
698:
610:
573:
1144:
673:
oxidizes exclusively at the benzylic positions to give
220:
is referred to as a "benzylic" carbocation. The benzyl
1920:
686:
300:
Benzyl is most commonly abbreviated Bn. For example,
1893:
1760:
1591:
Chandler), Norman, R. O. C. (Richard Oswald (1993).
1544:. Schore, Neil Eric, 1948- (8e ed.). New York.
2207:
844:
240:. The benzyl cation or phenylcarbenium ion is the
1417:(5th ed.). New York, NY: Springer. pp.
335:
264:; the benzyl anion or phenylmethanide ion is the
3222:
1787:
1956:
1722:Greene's Protective Groups in Organic Synthesis
1720:Wuts, Peter G. M.; Greene, Theodora W. (2006).
1542:Organic chemistry : structure and function
344:for benzylic C−H bonds. Specifically, the bond
96:or molecular fragment possessing the structure
1640:Encyclopedia of Reagents for Organic Synthesis
1508:Ullmann's Encyclopedia of Industrial Chemistry
1441:
2277:
2059:-Nucleoside from Streptomyces malaysiensis".
1476:
1408:
2163:
1623:: CS1 maint: multiple names: authors list (
1959:"Total Synthesis of (−)-Pseudolaric Acid B"
1576:) CS1 maint: multiple names: authors list (
1277:The benzyl group is occasionally used as a
1204:-methoxybenzyl halide (chloride or bromide)
68:, and benzyl methyl ether. R = heteroatom,
2284:
2270:
1719:
1669:
1572:: CS1 maint: location missing publisher (
859:Functionalization at the benzylic position
2031:
1990:
1590:
1355:
1244:
1003:(DMF) at ambient to elevated temperatures
824:
746:
442:one of the strongest aliphatic C−H bonds
292:and may exist as reactive intermediates.
2101:
1963:Journal of the American Chemical Society
1672:Journal of the American Chemical Society
1637:
1480:Journal of the American Chemical Society
1371:
1178:
931:
44:
2016:"Asymmetric Total Synthesis of Taxol\R"
1019:
14:
3223:
2201:
1724:(4th ed.). Wiley Online Library.
1540:C., Vollhardt, K. Peter (2018-01-29).
1409:Carey, F. A.; Sundberg, R. J. (2008).
2291:
2265:
940:
867:will brominate a benzylic C–H bond: (
1715:
1713:
1711:
1709:
416:such bonds show enhanced reactivity
177:. Benzyl is not to be confused with
1504:
1470:
1272:
1024:Benzyl ethers can be removed under
572:more activated vs diphenylmethyle (
24:
1539:
1343:
1311:
1257:
1230:
1052:
976:
509:comparable to vinyl radical, rare
25:
3247:
2231:
1706:
1380:with H atoms omitted for clarity.
2249:
2238:
2104:The Journal of Organic Chemistry
1924:The Journal of Organic Chemistry
1790:The Journal of Organic Chemistry
295:
2157:
2130:
2095:
2048:
2007:
1950:
1914:
1887:
1843:
1816:
1781:
1754:
1593:Principles of organic synthesis
1149:-methoxybenzyl protecting group
885:) or concentrated nitric acid (
141:
2020:Chemistry – A European Journal
1663:
1631:
1584:
1533:
1498:
1435:
1402:
760:
336:Reactivity of benzylic centers
13:
1:
2151:10.1016/S0040-4020(01)85435-1
1908:10.1016/S0040-4039(00)87907-1
1775:10.1016/S0040-4039(00)96491-8
1396:
1221:Trifluoromethanesulfonic acid
1192:Strong base such as powdered
1063:Single electron process with
1837:10.1016/j.tetlet.2003.09.106
1176:is used to protect thiols).
928:in DMSO performs similarly.
900:). Finally, the complex of
50:Benzyl group and derivatives
7:
1642:, American Cancer Society,
1456:10.1021/acs.chemrev.6b00664
1384:
80:etc. or other substituents.
10:
3252:
29:
27:Chemical group (–CH₂–C₆H₅)
3179:
3138:
3058:
3035:
2997:
2974:
2869:
2790:
2660:
2637:
2593:
2536:
2459:
2434:
2299:
1250:2,3-Dichloro-5,6-dicyano-
1217:) in toluene at 0 °C
659:free radical halogenation
414:akin to allylic C−H bonds
374:
372:Bond-dissociation energy
371:
368:
365:
169:substituent, for example
1648:10.1002/047084289x.rc170
1517:10.1002/14356007.a26_193
1289:. Other methods exist.
539:similar to benzylic C-H
342:bond dissociation energy
30:Not to be confused with
3190:chemical classification
1511:. Weinheim: Wiley-VCH.
1364:in the presence of the
1211:Scandium (III) triflate
52:: Benzyl group, benzyl
2254:Quotations related to
1381:
1348:
1316:
1262:
1235:
1188:
1099:at ambient temperature
1057:
991:can be achieved using
981:
846:
81:
3197:chemical nomenclature
1375:
1347:
1315:
1261:
1234:
1182:
1056:
980:
932:As a protecting group
865:Wohl-Ziegler reaction
847:
472:slightly weaker than
48:
2245:Chemistry portal
1378:tetrabenzylzirconium
1356:Deprotection methods
1254:-benzoquinone (DDQ)
1245:Deprotection methods
1187:-methoxybenzyl group
1174:4-Methoxybenzylthiol
1020:Deprotection methods
926:2-iodoxybenzoic acid
906:3,5-dimethylpyrazole
684:
111:. Benzyl features a
66:benzyl chloroformate
2653:not C, H or O)
2116:10.1021/jo00078a030
1969:(47): 14556–14557.
1936:10.1021/jo00175a010
1896:Tetrahedron Letters
1825:Tetrahedron Letters
1802:10.1021/jo00220a058
1763:Tetrahedron Letters
1492:10.1021/ja00051a010
1297:and benzyl halide (
1295:potassium carbonate
1194:potassium hydroxide
987:Monobenzylation of
965:and benzyl halide (
959:potassium hydroxide
837:
814:
801:
788:
775:
759:
739:
726:
713:
700:
609:"doubly benzylic" (
290:reaction mechanisms
200:ring. For example,
3095:Hypervalent iodine
1730:10.1002/0470053488
1382:
1349:
1317:
1263:
1236:
1189:
1058:
982:
941:Alcohol protection
842:
825:
802:
789:
776:
763:
747:
727:
714:
701:
688:
647:"triply benzylic"
405:benzylic C−H bond
332:, abbreviated Ph.
148:IUPAC nomenclature
82:
3236:Protecting groups
3218:
3217:
3156:Sulfenyl chloride
3134:
3133:
2633:
2632:
2452:(only C, H and O)
2293:Functional groups
2222:10.1021/om300820b
2216:(23): 8208–8217.
2179:10.1021/ol0362212
2110:(26): 7490–7497.
2073:10.1021/ol030095k
2067:(23): 4277–4280.
1975:10.1021/ja076165q
1902:(20): 2459–2462.
1865:10.1021/ol034037a
1831:(46): 8483–8485.
1796:(20): 3940–3942.
1769:(37): 4303–4306.
1684:10.1021/ja004218x
1678:(13): 3183–3185.
1486:(25): 9787–9792.
1450:(13): 8622–8648.
1287:organic synthesis
1170:organic synthesis
1111:-Bromosuccinimide
1038:, and the use of
1001:dimethylformamide
902:chromium trioxide
840:
828:
817:
805:
792:
779:
766:
750:
730:
717:
704:
691:
675:terephthalic acid
651:
650:
268:with the formula
181:with the formula
86:organic chemistry
16:(Redirected from
3243:
3185:
3090:Trifluoromethoxy
2658:
2657:
2654:
2457:
2456:
2453:
2306:
2286:
2279:
2272:
2263:
2262:
2253:
2243:
2242:
2241:
2226:
2225:
2205:
2199:
2198:
2161:
2155:
2154:
2134:
2128:
2127:
2099:
2093:
2092:
2052:
2046:
2045:
2035:
2011:
2005:
2004:
1994:
1954:
1948:
1947:
1918:
1912:
1911:
1891:
1885:
1884:
1847:
1841:
1840:
1820:
1814:
1813:
1785:
1779:
1778:
1758:
1752:
1751:
1717:
1704:
1703:
1667:
1661:
1660:
1635:
1629:
1628:
1622:
1614:
1588:
1582:
1581:
1571:
1563:
1537:
1531:
1530:
1502:
1496:
1495:
1474:
1468:
1467:
1444:Chemical Reviews
1439:
1433:
1432:
1416:
1406:
1279:protecting group
1273:Amine protection
1162:protecting group
1156:-Methoxybenzyl (
1120:-Iodosuccinimide
1007:Primary alcohols
951:carboxylic acids
923:
915:
899:
891:
884:
877:
851:
849:
848:
843:
841:
838:
836:
833:
826:
815:
813:
810:
803:
800:
797:
790:
787:
784:
777:
774:
771:
764:
758:
755:
748:
738:
735:
728:
725:
722:
715:
712:
709:
702:
699:
696:
689:
632:
595:
558:
524:
494:
479:
457:
427:
402:
363:
362:
359:
331:
319:
287:
286:
285:
282:
263:
262:
261:
258:
239:
224:has the formula
219:
191:
168:
137:
126:) attached to a
125:
110:
51:
21:
3251:
3250:
3246:
3245:
3244:
3242:
3241:
3240:
3221:
3220:
3219:
3214:
3183:
3175:
3130:
3085:Trichloromethyl
3080:Trifluoromethyl
3054:
3031:
2993:
2970:
2865:
2834:Phosphine oxide
2786:
2652:
2650:
2649:
2647:
2645:
2643:
2641:
2639:
2629:
2589:
2532:
2451:
2450:
2445:
2440:
2430:
2304:
2303:
2295:
2290:
2239:
2237:
2234:
2229:
2210:Organometallics
2206:
2202:
2167:Organic Letters
2162:
2158:
2135:
2131:
2100:
2096:
2061:Organic Letters
2053:
2049:
2012:
2008:
1955:
1951:
1919:
1915:
1892:
1888:
1853:Organic Letters
1848:
1844:
1821:
1817:
1786:
1782:
1759:
1755:
1740:
1718:
1707:
1668:
1664:
1658:
1636:
1632:
1616:
1615:
1603:
1589:
1585:
1565:
1564:
1552:
1538:
1534:
1527:
1503:
1499:
1475:
1471:
1440:
1436:
1429:
1421:–808, 312–313.
1407:
1403:
1399:
1387:
1358:
1334:
1275:
1247:
1225:dichloromethane
1216:
1160:) is used as a
1151:
1138:dichloromethane
1135:
1093:
1081:
1072:
1022:
1014:
996:
943:
934:
921:
917:
913:
909:
897:
893:
890:
886:
883:
879:
876:
872:
868:
861:
834:
829:
811:
806:
798:
793:
785:
780:
772:
767:
756:
751:
736:
731:
723:
718:
710:
705:
697:
692:
687:
685:
682:
681:
630:
626:
622:
618:
593:
589:
585:
581:
556:
552:
548:
544:
522:
518:
514:
492:
488:
484:
477:
473:
455:
451:
447:
425:
421:
415:
400:
396:
392:
388:
357:
353:
349:
345:
338:
329:
325:
321:
317:
313:
309:
298:
283:
280:
279:
277:
273:
269:
259:
256:
255:
253:
249:
245:
237:
233:
229:
225:
217:
213:
209:
205:
201:
190:
186:
182:
175:benzyl benzoate
171:benzyl chloride
167:
163:
159:
155:
144:
135:
131:
128:methylene group
124:
120:
116:
109:
105:
101:
97:
49:
43:
28:
23:
22:
15:
12:
11:
5:
3249:
3239:
3238:
3233:
3216:
3215:
3213:
3212:
3211:
3210:
3205:
3193:
3186:
3180:
3177:
3176:
3174:
3173:
3171:Sulfinylamines
3168:
3163:
3158:
3153:
3151:Phosphoramides
3148:
3146:Isothiocyanate
3142:
3140:
3136:
3135:
3132:
3131:
3129:
3128:
3123:
3122:
3121:
3111:
3110:
3109:
3099:
3098:
3097:
3092:
3087:
3082:
3077:
3066:
3064:
3056:
3055:
3053:
3052:
3047:
3041:
3039:
3033:
3032:
3030:
3029:
3024:
3022:Selenenic acid
3019:
3017:Seleninic acid
3014:
3012:Selenonic acid
3009:
3003:
3001:
2995:
2994:
2992:
2991:
2986:
2980:
2978:
2972:
2971:
2969:
2968:
2963:
2958:
2953:
2948:
2943:
2938:
2933:
2928:
2923:
2918:
2913:
2908:
2903:
2898:
2893:
2892:
2891:
2881:
2875:
2873:
2867:
2866:
2864:
2863:
2858:
2853:
2848:
2847:
2846:
2836:
2831:
2826:
2821:
2820:
2819:
2809:
2808:
2807:
2805:Phosphodiester
2796:
2794:
2788:
2787:
2785:
2784:
2779:
2774:
2769:
2764:
2759:
2754:
2749:
2744:
2739:
2734:
2729:
2724:
2719:
2714:
2709:
2704:
2699:
2694:
2689:
2684:
2683:
2682:
2677:
2666:
2664:
2655:
2651:(one element,
2635:
2634:
2631:
2630:
2628:
2627:
2626:
2625:
2615:
2614:
2613:
2608:
2597:
2595:
2591:
2590:
2588:
2587:
2582:
2577:
2576:
2575:
2565:
2564:
2563:
2558:
2553:
2542:
2540:
2534:
2533:
2531:
2530:
2528:Methylenedioxy
2525:
2520:
2519:
2518:
2513:
2503:
2502:
2501:
2496:
2486:
2485:
2484:
2474:
2469:
2463:
2461:
2454:
2432:
2431:
2429:
2428:
2423:
2418:
2417:
2416:
2411:
2401:
2400:
2399:
2394:
2389:
2384:
2379:
2374:
2364:
2363:
2362:
2357:
2347:
2346:
2345:
2340:
2335:
2330:
2325:
2320:
2309:
2307:
2305:(only C and H)
2297:
2296:
2289:
2288:
2281:
2274:
2266:
2260:
2259:
2247:
2233:
2232:External links
2230:
2228:
2227:
2200:
2173:(2): 249–252.
2156:
2145:(2): 523–544.
2129:
2094:
2047:
2026:(1): 121–161.
2006:
1949:
1913:
1886:
1859:(5): 761–764.
1842:
1815:
1780:
1753:
1738:
1705:
1662:
1656:
1630:
1602:978-0751401264
1601:
1583:
1550:
1532:
1526:978-3527306732
1525:
1497:
1469:
1434:
1427:
1400:
1398:
1395:
1394:
1393:
1386:
1383:
1370:
1369:
1357:
1354:
1353:
1352:
1351:
1350:
1332:
1320:
1319:
1318:
1274:
1271:
1270:
1269:
1266:
1265:
1264:
1246:
1243:
1242:
1241:
1240:
1239:
1238:
1237:
1218:
1214:
1205:
1198:sodium hydride
1150:
1143:
1142:
1141:
1133:
1130:Trimethylsilyl
1126:
1125:
1124:
1123:
1114:
1105:
1100:
1091:
1083:
1082:
1079:
1070:
1061:
1060:
1059:
1048:hydrogenolysis
1046:Removed using
1021:
1018:
1017:
1016:
1012:
1004:
994:
985:
984:
983:
963:sodium hydride
942:
939:
933:
930:
919:
911:
895:
888:
881:
874:
870:
860:
857:
853:
852:
832:
823:
820:
809:
796:
783:
770:
762:
754:
745:
742:
734:
721:
708:
695:
663:hydrogenolysis
649:
648:
645:
642:
639:
633:
628:
624:
620:
615:
614:
607:
605:
602:
599:diphenylmethyl
596:
591:
587:
583:
578:
577:
570:
568:
565:
559:
554:
550:
546:
541:
540:
537:
534:
531:
525:
520:
516:
511:
510:
507:
504:
501:
495:
490:
486:
481:
480:
475:
470:
467:
464:
458:
453:
449:
444:
443:
440:
437:
434:
428:
423:
418:
417:
412:
409:
406:
403:
398:
394:
390:
385:
384:
381:
377:
376:
373:
370:
367:
355:
351:
347:
337:
334:
327:
323:
315:
311:
302:benzyl alcohol
297:
294:
275:
271:
251:
247:
235:
231:
227:
215:
211:
207:
203:
188:
184:
165:
161:
157:
143:
140:
133:
122:
118:
107:
103:
99:
62:benzyl bromide
26:
9:
6:
4:
3:
2:
3248:
3237:
3234:
3232:
3229:
3228:
3226:
3209:
3206:
3204:
3201:
3200:
3199:
3198:
3194:
3192:
3191:
3187:
3182:
3181:
3178:
3172:
3169:
3167:
3164:
3162:
3159:
3157:
3154:
3152:
3149:
3147:
3144:
3143:
3141:
3137:
3127:
3124:
3120:
3117:
3116:
3115:
3112:
3108:
3105:
3104:
3103:
3100:
3096:
3093:
3091:
3088:
3086:
3083:
3081:
3078:
3076:
3073:
3072:
3071:
3068:
3067:
3065:
3063:
3062:
3057:
3051:
3050:Telluroketone
3048:
3046:
3043:
3042:
3040:
3038:
3034:
3028:
3025:
3023:
3020:
3018:
3015:
3013:
3010:
3008:
3005:
3004:
3002:
3000:
2996:
2990:
2987:
2985:
2982:
2981:
2979:
2977:
2973:
2967:
2964:
2962:
2959:
2957:
2954:
2952:
2949:
2947:
2944:
2942:
2939:
2937:
2936:Sulfonic acid
2934:
2932:
2929:
2927:
2926:Sulfinic acid
2924:
2922:
2921:Thiosulfonate
2919:
2917:
2914:
2912:
2911:Thiosulfinate
2909:
2907:
2906:Sulfenic acid
2904:
2902:
2899:
2897:
2894:
2890:
2887:
2886:
2885:
2882:
2880:
2877:
2876:
2874:
2872:
2868:
2862:
2861:Phosphaallene
2859:
2857:
2856:Phosphaalkyne
2854:
2852:
2851:Phosphaalkene
2849:
2845:
2842:
2841:
2840:
2837:
2835:
2832:
2830:
2827:
2825:
2822:
2818:
2815:
2814:
2813:
2810:
2806:
2803:
2802:
2801:
2798:
2797:
2795:
2793:
2789:
2783:
2780:
2778:
2775:
2773:
2770:
2768:
2765:
2763:
2760:
2758:
2755:
2753:
2750:
2748:
2745:
2743:
2740:
2738:
2735:
2733:
2730:
2728:
2725:
2723:
2720:
2718:
2715:
2713:
2710:
2708:
2705:
2703:
2700:
2698:
2695:
2693:
2690:
2688:
2685:
2681:
2678:
2676:
2673:
2672:
2671:
2668:
2667:
2665:
2663:
2659:
2656:
2636:
2624:
2621:
2620:
2619:
2616:
2612:
2609:
2607:
2604:
2603:
2602:
2599:
2598:
2596:
2592:
2586:
2583:
2581:
2578:
2574:
2571:
2570:
2569:
2566:
2562:
2559:
2557:
2554:
2552:
2549:
2548:
2547:
2544:
2543:
2541:
2539:
2535:
2529:
2526:
2524:
2523:Ethylenedioxy
2521:
2517:
2514:
2512:
2509:
2508:
2507:
2504:
2500:
2497:
2495:
2492:
2491:
2490:
2487:
2483:
2480:
2479:
2478:
2475:
2473:
2470:
2468:
2465:
2464:
2462:
2458:
2455:
2449:
2443:
2438:
2433:
2427:
2424:
2422:
2419:
2415:
2412:
2410:
2407:
2406:
2405:
2402:
2398:
2395:
2393:
2390:
2388:
2385:
2383:
2380:
2378:
2375:
2373:
2370:
2369:
2368:
2365:
2361:
2358:
2356:
2353:
2352:
2351:
2348:
2344:
2341:
2339:
2336:
2334:
2331:
2329:
2326:
2324:
2321:
2319:
2316:
2315:
2314:
2311:
2310:
2308:
2302:
2298:
2294:
2287:
2282:
2280:
2275:
2273:
2268:
2267:
2264:
2257:
2252:
2248:
2246:
2236:
2235:
2223:
2219:
2215:
2211:
2204:
2196:
2192:
2188:
2184:
2180:
2176:
2172:
2168:
2160:
2152:
2148:
2144:
2140:
2133:
2125:
2121:
2117:
2113:
2109:
2105:
2098:
2090:
2086:
2082:
2078:
2074:
2070:
2066:
2062:
2058:
2051:
2043:
2039:
2034:
2029:
2025:
2021:
2017:
2010:
2002:
1998:
1993:
1988:
1984:
1980:
1976:
1972:
1968:
1964:
1960:
1953:
1945:
1941:
1937:
1933:
1929:
1925:
1917:
1909:
1905:
1901:
1897:
1890:
1882:
1878:
1874:
1870:
1866:
1862:
1858:
1854:
1846:
1838:
1834:
1830:
1826:
1819:
1811:
1807:
1803:
1799:
1795:
1791:
1784:
1776:
1772:
1768:
1764:
1757:
1749:
1745:
1741:
1739:9780470053485
1735:
1731:
1727:
1723:
1716:
1714:
1712:
1710:
1701:
1697:
1693:
1689:
1685:
1681:
1677:
1673:
1666:
1659:
1657:9780470842898
1653:
1649:
1645:
1641:
1634:
1626:
1620:
1612:
1608:
1604:
1598:
1594:
1587:
1579:
1575:
1569:
1561:
1557:
1553:
1551:9781319079451
1547:
1543:
1536:
1528:
1522:
1518:
1514:
1510:
1509:
1501:
1493:
1489:
1485:
1481:
1473:
1465:
1461:
1457:
1453:
1449:
1445:
1438:
1430:
1428:9780387448978
1424:
1420:
1415:
1414:
1405:
1401:
1392:
1389:
1388:
1379:
1376:Structure of
1374:
1367:
1363:
1362:Hydrogenation
1360:
1359:
1346:
1342:
1341:
1340:
1336:
1328:
1324:
1321:
1314:
1310:
1309:
1308:
1304:
1300:
1296:
1292:
1291:
1290:
1288:
1284:
1280:
1267:
1260:
1256:
1255:
1253:
1249:
1248:
1233:
1229:
1228:
1227:at 0 °C
1226:
1222:
1219:
1212:
1209:
1208:
1206:
1203:
1199:
1195:
1191:
1190:
1186:
1181:
1177:
1175:
1171:
1167:
1163:
1159:
1155:
1148:
1139:
1131:
1128:
1127:
1121:
1119:
1115:
1112:
1110:
1106:
1104:
1101:
1098:
1094:
1088:
1087:
1085:
1084:
1077:
1073:
1066:
1062:
1055:
1051:
1050:
1049:
1045:
1044:
1043:
1041:
1037:
1035:
1030:
1028:
1015:
1008:
1005:
1002:
998:
990:
986:
979:
975:
974:
972:
968:
964:
960:
956:
955:
954:
952:
948:
938:
929:
927:
922:R → ArC(O)R
907:
903:
866:
856:
830:
821:
818:
807:
794:
781:
768:
752:
743:
740:
732:
719:
706:
693:
680:
679:
678:
676:
672:
668:
664:
660:
656:
646:
643:
640:
637:
634:
617:
616:
612:
608:
606:
603:
600:
597:
580:
579:
575:
571:
569:
566:
563:
560:
543:
542:
538:
535:
532:
529:
526:
513:
512:
508:
505:
502:
499:
496:
483:
482:
471:
468:
465:
462:
459:
446:
445:
441:
438:
435:
432:
429:
420:
419:
413:
410:
407:
404:
387:
386:
382:
379:
378:
364:
361:
343:
333:
307:
303:
296:Abbreviations
293:
291:
267:
244:with formula
243:
223:
199:
195:
180:
176:
172:
153:
150:, the prefix
149:
139:
129:
114:
95:
91:
87:
79:
75:
71:
67:
63:
59:
55:
47:
41:
37:
33:
19:
3195:
3188:
3102:Vinyl halide
3059:
2989:Borinic acid
2984:Boronic acid
2961:Thioxanthate
2413:
2301:Hydrocarbons
2258:at Wikiquote
2256:Benzyl group
2213:
2209:
2203:
2170:
2166:
2159:
2142:
2138:
2132:
2107:
2103:
2097:
2064:
2060:
2056:
2050:
2023:
2019:
2009:
1966:
1962:
1952:
1930:(1): 51–56.
1927:
1923:
1916:
1899:
1895:
1889:
1856:
1852:
1845:
1828:
1824:
1818:
1793:
1789:
1783:
1766:
1762:
1756:
1721:
1675:
1671:
1665:
1639:
1633:
1592:
1586:
1541:
1535:
1506:
1500:
1483:
1479:
1472:
1447:
1443:
1437:
1412:
1404:
1323:Benzaldehyde
1276:
1251:
1201:
1184:
1157:
1153:
1152:
1146:
1117:
1108:
1039:
1032:
1025:
1023:
944:
935:
862:
854:
666:
652:
339:
299:
222:free radical
193:
192:. The term
154:refers to a
151:
145:
142:Nomenclature
89:
83:
58:benzyl amine
3231:Aryl groups
3166:Thiocyanate
3161:Sulfonamide
3126:Perchlorate
3114:Acyl halide
3075:Fluoroethyl
2956:Thionoester
2844:Phosphonium
2829:Phosphinate
2824:Phosphonous
2812:Phosphonate
2511:Hydroperoxy
2333:Cyclopropyl
2139:Tetrahedron
1391:Benzylamine
1325:, 6 M
1097:acetic acid
1040:Lewis acids
380:(kcal/mol)
242:carbocation
94:substituent
3225:Categories
3070:Haloalkane
2941:Thioketone
2896:Persulfide
2792:Phosphorus
2757:Isocyanate
2747:Isonitrile
2648:or oxygen
2646:hydrogen,
2642:not being
2623:Orthoester
2516:Dioxiranes
2494:Enol ether
2382:1-Propenyl
1560:1007924903
1397:References
1223:(TfOH) in
1132:iodide (Me
1036:conditions
1029:conditions
3203:inorganic
3037:Tellurium
2951:Thioester
2916:Sulfoxide
2901:Disulfide
2889:Sulfonium
2839:Phosphine
2817:Phosphite
2800:Phosphate
2732:Carbamate
2707:Hydrazone
2640:element,
2638:Only one
2611:Anhydride
2350:Methylene
2187:1523-7060
2124:0022-3263
2081:1523-7060
2042:1521-3765
1983:0002-7863
1944:0022-3263
1873:1523-7060
1810:0022-3263
1692:0002-7863
1619:cite book
1568:cite book
1366:palladium
1034:oxidative
1027:reductive
898:→ ArCOOH
873:→ ArCBrR
761:⟶
655:oxidation
638:C–H bond
601:C–H bond
564:C–H bond
562:fluorenyl
530:C–H bond
500:C−H bond
463:C−H bond
433:C−H bond
383:(kJ/mol)
266:carbanion
138:) group.
3184:See also
3119:Chloride
3045:Tellurol
2999:Selenium
2966:Xanthate
2680:Ammonium
2662:Nitrogen
2644:carbon,
2601:Carboxyl
2568:Aldehyde
2556:Acryloyl
2538:carbonyl
2442:hydrogen
2397:Cumulene
2195:14723540
2089:14601979
2001:17985906
1881:12605509
1748:83393227
1700:11457049
1611:27813843
1464:28281752
1385:See also
1368:catalyst
1339:methanol
1307:methanol
1293:Aqueous
1213:(Sc(OTf)
1166:alcohols
1136:SiI) in
1011:Cu(acac)
947:alcohols
613:= 32.2)
576:= 22.6)
375:Comment
198:aromatic
194:benzylic
3208:organic
3007:Selenol
2931:Sulfone
2884:Sulfide
2782:NONOate
2777:Nitroso
2767:Nitrite
2762:Nitrate
2752:Cyanate
2742:Nitrile
2727:Amidine
2722:Imidate
2692:Nitrene
2687:Hydrazo
2675:Enamine
2606:Acetoxy
2594:carboxy
2561:Benzoyl
2499:Epoxide
2482:Methoxy
2472:Alcohol
2426:Carbene
2360:Methine
1992:2535803
528:allylic
306:benzoyl
113:benzene
92:is the
54:radical
36:benzoyl
3107:Iodide
3027:Selone
2871:Sulfur
2580:Ketone
2573:Ketene
2551:Acetyl
2506:Peroxy
2477:Alkoxy
2467:Acetal
2448:oxygen
2437:carbon
2421:Alkyne
2414:Benzyl
2409:Phenyl
2392:Allene
2387:Crotyl
2367:Alkene
2355:Bridge
2343:Pentyl
2328:Propyl
2318:Methyl
2193:
2185:
2122:
2087:
2079:
2040:
1999:
1989:
1981:
1942:
1879:
1871:
1808:
1746:
1736:
1698:
1690:
1654:
1609:
1599:
1558:
1548:
1523:
1462:
1425:
1283:amines
914:−dmpyz
671:xylene
636:trityl
498:phenyl
431:methyl
308:group
179:phenyl
152:benzyl
115:ring (
90:benzyl
40:phenyl
32:benzil
18:Benzyl
3139:Other
2976:Boron
2946:Thial
2879:Thiol
2772:Nitro
2737:Imide
2717:Amide
2702:Oxime
2697:Imine
2670:Amine
2618:Ester
2585:Ynone
2489:Ether
2460:R-O-R
2435:Only
2377:Allyl
2372:Vinyl
2338:Butyl
2323:Ethyl
2313:Alkyl
1744:S2CID
1305:) in
1122:(NIS)
1113:(NBS)
1103:Ozone
989:diols
894:ArCHR
869:ArCHR
661:, or
519:=CHCH
461:ethyl
369:Bond
366:Bond
318:C(O)−
78:allyl
70:alkyl
38:, or
3061:Halo
2546:Acyl
2446:and
2404:Aryl
2191:PMID
2183:ISSN
2120:ISSN
2085:PMID
2077:ISSN
2038:ISSN
1997:PMID
1979:ISSN
1940:ISSN
1877:PMID
1869:ISSN
1806:ISSN
1734:ISBN
1696:PMID
1688:ISSN
1652:ISBN
1625:link
1607:OCLC
1597:ISBN
1578:link
1574:link
1556:OCLC
1546:ISBN
1521:ISBN
1460:PMID
1423:ISBN
1331:NaBH
1329:and
1303:BnBr
1299:BnCl
1281:for
1200:and
1183:The
1164:for
1145:The
971:BnBr
967:BnCl
949:and
918:ArCH
904:and
892:): (
880:KMnO
644:339
594:CH−H
557:CH−H
536:372
506:473
503:113
469:423
466:101
439:439
436:105
411:377
210:)(CH
98:R−CH
74:aryl
2712:Azo
2218:doi
2175:doi
2147:doi
2112:doi
2069:doi
2028:doi
1987:PMC
1971:doi
1967:129
1932:doi
1904:doi
1861:doi
1833:doi
1798:doi
1771:doi
1726:doi
1680:doi
1676:123
1644:doi
1513:doi
1488:doi
1484:114
1452:doi
1448:117
1419:806
1337:in
1327:HCl
1285:in
1196:or
1168:in
1158:PMB
1090:CrO
1078:/NH
1074:or
999:in
969:or
961:or
924:).
910:CrO
887:HNO
641:81
631:C−H
611:pKa
604:82
574:pKa
567:80
533:89
478:C−H
426:C−H
408:90
173:or
146:In
132:−CH
84:In
3227::
2444:,
2439:,
2214:31
2212:.
2189:.
2181:.
2169:.
2143:46
2141:.
2118:.
2108:58
2106:.
2083:.
2075:.
2063:.
2036:.
2022:.
2018:.
1995:.
1985:.
1977:.
1965:.
1961:.
1938:.
1928:49
1926:.
1900:29
1898:.
1875:.
1867:.
1855:.
1829:44
1827:.
1804:.
1794:50
1792:.
1767:28
1765:.
1742:.
1732:.
1708:^
1694:.
1686:.
1674:.
1650:,
1621:}}
1617:{{
1605:.
1570:}}
1566:{{
1554:.
1519:.
1482:.
1458:.
1446:.
1335:CN
1301:,
1076:Li
1069:NH
1065:Na
1042:.
1031:,
993:Ag
973:)
953:.
804:CO
778:CC
765:HO
729:CH
690:CH
677::
657:,
619:(C
582:(C
545:(C
523:−H
515:CH
493:−H
456:−H
401:−H
397:CH
358:−H
354:CH
278:CH
254:CH
234:CH
202:(C
164:CH
102:−C
88:,
76:,
72:,
64:,
60:,
56:,
34:,
2285:e
2278:t
2271:v
2224:.
2220::
2197:.
2177::
2171:6
2153:.
2149::
2126:.
2114::
2091:.
2071::
2065:5
2057:C
2044:.
2030::
2024:5
2003:.
1973::
1946:.
1934::
1910:.
1906::
1883:.
1863::
1857:5
1839:.
1835::
1812:.
1800::
1777:.
1773::
1750:.
1728::
1702:.
1682::
1646::
1627:)
1613:.
1580:)
1562:.
1529:.
1515::
1494:.
1490::
1466:.
1454::
1431:.
1333:3
1252:p
1215:3
1202:p
1185:p
1172:(
1154:p
1147:p
1134:3
1118:N
1109:N
1095:/
1092:3
1080:3
1071:3
1067:/
1013:2
997:O
995:2
920:2
912:3
908:(
896:2
889:3
882:4
875:2
871:2
839:O
831:2
827:H
822:2
819:+
816:H
808:2
795:4
791:H
782:6
769:2
753:2
749:O
744:3
741:+
733:3
720:4
716:H
707:6
703:C
694:3
669:-
667:p
629:3
627:)
625:5
623:H
621:6
592:2
590:)
588:5
586:H
584:6
555:2
553:)
551:4
549:H
547:6
521:2
517:2
491:5
489:H
487:6
485:C
476:3
474:H
454:5
452:H
450:2
448:C
424:3
422:H
399:2
395:5
393:H
391:6
389:C
356:2
352:5
350:H
348:6
346:C
330:−
328:5
326:H
324:6
322:C
316:5
314:H
312:6
310:C
284:2
281:−
276:5
274:H
272:6
270:C
260:2
257:+
252:5
250:H
248:6
246:C
238:•
236:2
232:5
230:H
228:6
226:C
218:C
216:2
214:)
212:3
208:5
206:H
204:6
189:5
187:H
185:6
183:C
166:2
162:5
160:H
158:6
156:C
136:−
134:2
130:(
123:6
121:H
119:6
117:C
108:5
106:H
104:6
100:2
42:.
20:)
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.